An interdisciplinary team of researchers at Vanderbilt University has analyzed the simplest known biological clock and figured out what makes it tick. The results of their analysis are published in the March 27 issue of the journal Public Library of Science Biology.
Biological clocks are microscopic pacemakers. They are found in everything from pond scum to human beings and appear to help organize a dizzying array of biochemical processes. A traveler experiences jet lag when his or her internal clock becomes out of synch with the environment. Seasonal Affective Disorder, some types of depression, sleep disorders and problems adjusting to changes in work cycles all can occur when an individual's biological clock acts up. Recent studies have even found links between these molecular timepieces and cancer.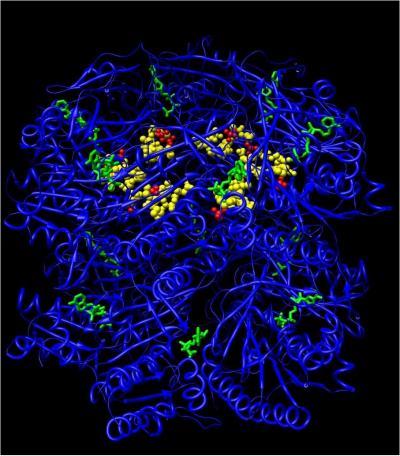 This illustration of KaiC shows the sites where ATP molecules (shown in green) attach and the location of the phosphorylation sites (shown in red). Credit: Carl Johnson Laboratory
This illustration of KaiC shows the sites where ATP molecules (shown in green) attach and the location of the phosphorylation sites (shown in red). Credit: Carl Johnson Laboratory
In 2005, a group of Japanese researchers surprised the scientific community by showing that the three proteins which make up the biological clock in blue green algae will establish a 24-hour cycle on their own when placed in a test tube with adenosine triphosphate (ATP), the chemical that powers biological reactions.
"That was a big surprise," says Carl Johnson, the professor of biological sciences who headed the new study. "We all thought the system was much more complicated and required feedback from the cell’s genetic machinery in order to work."
The announcement prompted Johnson, who had been working along similar lines, to assemble an interdisciplinary team to figure out how these three proteins can establish and maintain a steady, 24-hour cycle. He and his long-time collaborator, Professor of Biochemistry Martin Egli, recruited a group of researchers who are experts in electron microscopy – Associate Professor Phoebe Stewart and Research Fellow Dewight Williams – and biophysics – Professor Hassane Mchaourab – all from the Department of Molecular Physiology. Biomathematician Mark Byrne, a research fellow in pharmacology, rounded out the group.
Although the biological clock consists of only three basic parts – proteins that have been labeled KaiA, KaiB and KaiC – when they began analyzing what was taking place in the test tube they discovered a lot more was going on than they had imagined.
"The coolest part is that a simple biological machine can do such an astounding thing as keeping time," says Williams. "It is the most fascinating biological puzzle that I have come across in my career so far."
The basic question that the researchers set out to understand is how these molecules, which are undergoing reactions at a second-by-second and minute-by-minute frequency, can sustain a 24-hour cycle.
The largest cog in the bioclock is the protein KaiC. It is a large, barrel-shaped molecule assembled from six identical components. The diurnal cycle takes the form of the regular increase and decrease in the number of phosphate groups attached to the KaiC molecules. The attachment and detachment of phosphate groups – a process called phosphorylation and dephosphorylation – is a common method of protein regulation. When KaiC is phosphorylated it interacts in different ways with other proteins in the cell than it does when it is dephosphorylated. That allows the bioclock to turn various cellular processes on and off.
Based on previous research, Johnson and his colleagues had some insight into the role of the two smaller proteins. They knew that when KaiA binds to KaiC the phosphorylation rate increases, either by making it easier for phosphate groups to bind to the hexamer or making it more difficult for them to break away. KaiB, by contrast, doesn’t bind to KaiC until it is highly phosphorylated. But, when it does, KaiB counteracts the influence of KaiA.
At the outset, the researchers envisioned a relatively straightforward process: KaiA would bind with KaiC and phosphorylation would gradually increase for 12 hours. Then something would trigger KaiB to begin bonding with these complexes and the phosphorylation would gradually decrease for 12 hours. However, Johnson and Egli’s efforts to purify and crystallize the KaiAC and KaiABC complexes so they could determine their structure using X-ray crystallography repeatedly failed.
It wasn’t until they began putting the mixture under the transmission electron microscope that they realized the reason for this failure. "It turns out that the complexes do not form one static structure, which is why we could not crystallize them," says Stewart. "It doesn’t go from complex one to complex two three hours later and then three hours later to the next complex. Instead, you have mixtures of all different complexes at all time points, just in different ratios." The researchers divided the 24-hour cycle into seven equal phases: Starting at the lowest level of KaiC phosphorylation, in phases Up1 and Up2 the phosphorylation level increases until it reaches a peak level. Following this "P" phase, the hexamers begin dephosphorylating through phases Down1, Down2 and Down3, reaching its lowest level in the "T" phase (T for trough) and then it starts over.
The analysis also found that, in addition to KaiA, KaiB and KaiC, the test tube also contained large amounts of the three smaller molecules, called monomers, that are the basic building blocks for the bioclock proteins. KaiC is a hexamer that is made up of six monomers. KaiA is a dimer that is made up of two monomers. And KaiB is a tetramer that is made up of four monomers. At the same time that the three proteins are combining into complexes and breaking up again, KaiC is also breaking apart into monomers and then recombining.
While this provided a valuable new insight into the process, it did not explain what was actually going on. To help decipher the dynamics of this system, they turned to Byrne. "The task I was given by Carl was to figure out how this system of three proteins, when combined with ATP, can produce a 24-hour oscillation," says the biomathematician. "What we’ve come up with is our ‘best guess’ model for how the system works."
According to Byrne’s model, the key to the system’s stability is the role played by the exchange of monomers by KaiC hexamers. "The 24-hour cycle is the variation in the average phosphorylation level of the hexamers. To produce sustained rhythms in the system, you must have some way to synchronize the phosphorylation levels of individual hexamers," he says. The fact that the hexamers are exchanging monomers at a substantially faster rate than the process of phosphorylation and dephosphorylation keeps phosphorylation levels evenly distributed throughout the KaiC population. "If the population becomes asynchronous – that is, if some hexamers phosphorylate and dephosphorylate out of synch with the others – then the hexamers will start oscillating out of phase with each other and you will lose the rhythm."
The model successfully explains why a specific proportion of the three proteins is needed to establish the 24-hour rhythm, how temperature can reset the system and the general characteristics of the bioclock system. However, there is a great deal more to learn.
"This paper is our first step toward visualizing what is happening during the 24-hour cycle," says Stewart. "The next level of understanding will be how the proteins work together as a nanomachine to carry out their job."
Then, too, the researchers realize the way that bioclock systems work in living cells is substantially more complex than what takes place in a test tube. For one thing, there will be additional levels of regulation, such as control of the synthesis of the bioclock protein monomers, that influence their operation.
Written from a news release by Vanderbilt University.