Two unrelated studies on the Zika virus--one ruling out a theory for how Zika may be passing through the human placenta and another on using mouse models to trace Zika pathogenesis--appear April 5 in Cell Host & Microbe.
Trophoblasts Unlikely Placental Entry Point for Zika
One theory for how the Zika virus gets to the developing fetus is that it passes through the trophoblasts, a layer of placental cells that surround and nurture the fetus. But a collaborative team of virologists and reproductive scientists looking at cells isolated from full-term human placentas have found that Zika does not infect trophoblasts. How the virus is getting through the placenta is still unknown, but their work helps rule out an obvious pathway.
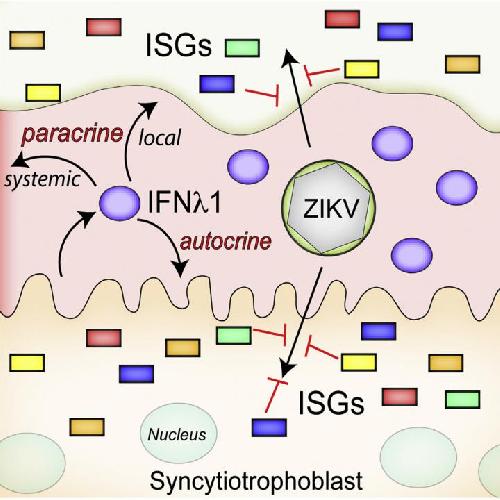 This visual abstract depicts the Bayer and Lennemann et al. finding that primary human placental trophoblasts are refractory to ZIKV infection due to their constitutive release of antiviral type III interferons, which also serves to protect nonplacental cells. These data suggest that rather than directly infecting the placenta, ZIKV likely uses alternative strategies to cross the placenta. Credit: Bayer and Lennemann et al./Cell Host & Microbe 2016
This visual abstract depicts the Bayer and Lennemann et al. finding that primary human placental trophoblasts are refractory to ZIKV infection due to their constitutive release of antiviral type III interferons, which also serves to protect nonplacental cells. These data suggest that rather than directly infecting the placenta, ZIKV likely uses alternative strategies to cross the placenta. Credit: Bayer and Lennemann et al./Cell Host & Microbe 2016
"The trophoblasts are the baby's first line of defense against anything that comes from the maternal blood, so you may expect these cells to have some way to resist viral infections," says Yoel Sadovsky, director of the Magee-Womens Research Institute at the University of Pittsburgh. "Based on our model, it seems that trophoblasts have an inherent capacity to resist Zika virus proliferation, although we have not ruled out other ways the virus can get into the fetal cavity."
Sadovsky, an expert in maternal-fetal medicine, has been working on the Zika-placenta connection with long-time collaborator Carolyn Coyne, at the University of Pittsburgh's Department of Microbiology and Molecular Genetics, an expert in RNA viruses, including flaviviruses--the family of viruses to which Zika belongs. Their examination of trophoblasts also revealed that human trophoblasts release a potent antiviral molecule called type III interferon, which stops the replication of Zika virus. (The researchers used two Zika strains in their study--one isolated from the Zika Forest in Uganda and another from Cambodia.)
"We really know shockingly little about how viruses cross the placenta--not just Zika but rubella, herpes, and other viruses that cause birth defects," Coyne says. "What makes our finding interesting is that these trophoblasts are potentially communicating with maternal cells to protect them against viral infections as well."
The strength of Coyne and Sadovsky's study is that the human trophoblasts cultured in the lab functioned nearly identically to those found in a developing placenta. One potential weakness of the study is that their trophoblasts were taken from third-trimester pregnancies, which means that trophoblasts could still be vulnerable to Zika virus during the first trimester. However, the researchers don't believe that it reduces the significance of their findings, as the virus seems to be transmitted throughout pregnancy.
With primary trophoblast infection ruled out, Coyne and Sadovsky will next use their placental cultures to explore other ways that viruses can reach a developing fetus.
Cell Host & Microbe, Bayer and Lennemann et al.: "Human Placental Trophoblasts Produce Type III Interferons that Confer Protection against Zika Virus Infection" http://dx.doi.org/10.1016/j.chom.2016.03.008. This project was supported by the National Institutes of Health and Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Awards.
Mouse Model of Zika Echoes Infection in Humans
Until recent weeks, only three papers using mice to study Zika infection had been published in the last 60 years. In some of these studies, viral particles were injected directly into the brain (which is not so clinically relevant), and so with the ongoing epidemic, Washington University School of Medicine researchers returned to the mouse model to find out which tissues are most vulnerable to Zika infection, this time after infection in the skin, which mimics inoculation by mosquito. There results show high numbers of virus in the rodent brain, spinal cord, and testes.
Early evidence suggests that Zika has trouble crossing into rodents. Zika doesn't seem to replicate in adult wild-type mice, so the investigators used animals genetically engineered without part of their antiviral immune systems (type I interferon) to generate a lethal infection.
"If you take away interferon, then the virus replicates quite well in the mouse and goes to the places that we see it causing disease in humans," says senior author Michael Diamond, who studies mosquito-borne viruses at the Washington University School of Medicine.
Diamond was inspired to pursue Zika after a meeting last June when Brazilian researchers described anecdotal evidence of a rise in infant birth defects and a local Zika outbreak. His laboratory, led by first author Helen Lazear, created the Zika mouse models and then looked for viral particles in tissues that related viruses (e.g., Dengue, West Nile) tend to infect. The biggest surprise came after they looked at the testes after mounting reports that Zika could be sexually transmitted.
"Viral levels were the highest that we saw in any tissues that we measured," Diamond says. "We are now doing subsequent tests to determine how long that may last."
Five strains of the virus were examined: the original strain from the Zika Forest in Ethiopia, three 1980s strains from Senegal, and a 2013 contemporary strain from French Polynesia. All yielded similar results, hinting that there may not be much difference in pathogenicity between individual strains, at least in this model.
Diamond is now using his models to explore how Zika responds to the mouse adaptive immune response--the cells that generate antibodies that tag and remove viruses.
Cell Host & Microbe, Lazear et al.: "A Mouse Model of Zika Virus Pathogenesis" http://dx.doi.org/10.1016/j.chom.2016.03.010. This work was supported by start-up funds from the University of North Carolina Department of Microbiology and Immunology and the Lineberger Comprehensive Cancer Center, as well as grants from the National Institutes of Health.
source: Cell Press