Reflecting the overall structural alterations in the tissue, changes in the flow of interstitial fluid in articular cartilage could be an indicator revealing the onset of osteoarthritis, according to a new study from the University of Eastern Finland.
The PhD study of Janne Mäkelä, MSc, focused on structural and functional changes in articular cartilage at different stages of osteoarthritis. By using finite element modelling, the study combined structural data with functional data measured from articular cartilage samples. This enabled a detailed analysis of how individual structural components affect cartilage loading, and how this process changes in the development of osteoarthritis.
Osteoarthritis is a disease for which no cure currently exists. It is a prevalent condition affecting and disabling an increasing group of people among the world's population, causing great costs to societies. Osteoarthritis also affects young people, for example as a result of an articular cartilage injury. The diagnosis of osteoarthritis is usually confirmed only after irreversible symptoms such as joint pain or joint stiffness have begun. Hip and knee replacement surgery is frequently used in order to maintain the patient's ability to remain physically active. Osteoarthritis can take several decades to develop, which offers a long time window to affect its development. Understanding the very early structural and functional changes, and the interplay between them, plays an important role in the diagnosis of osteoarthritis as well as in the development of treatment.
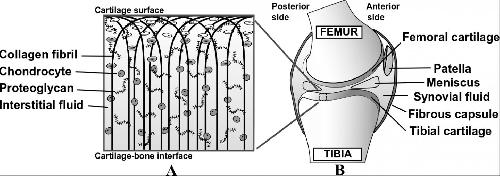 This is an illustration of the composition of articular cartilage (A) and a sagittal view of human knee joint (B). Credit: Janne Mäkelä
This is an illustration of the composition of articular cartilage (A) and a sagittal view of human knee joint (B). Credit: Janne Mäkelä
Computational modelling more accurate than traditional mechanical testing in detecting early changes in osteoarthritis
The role of articular cartilage is to serve as a shock absorber in the joint and to create a nearly friction-free surface for bones to slide freely. The function of articular cartilage is governed by three key structural components. Tissue fluid pressure ensures tissue stiffness under rapid cartilage loading such as jumping, at the same time as the collagen fibril network limits tissue deformations. Under static loading such as standing, tissue fluid flows out of the tissue, and negatively charged proteoglycan proteins ensure cartilage stiffness.
The study used microscopic and spectroscopic methods to analyse the structure of articular cartilage samples, enabling the creation of a detailed finite element model to replicate the data obtained by mechanical measurements. Finite element modelling was more sensitive method, compared to exclusively elastic mechanical analysis, in identifying functional changes in early osteoarthritis.
The study showed the flow of interstitial fluid to be associated with many osteoarthritis-induced structural changes. Damage to the solid parts of the cartilage enhances the fluid flow from the cartilage, weakening its stiffness under static loading. Increase in the fluid flow seems to be the first functional change indicative of osteoarthritis.
The study found that the collagen network architecture was the most susceptible single structural component to osteoarthritis-induced changes. Damage to the collagen network affects the shock absorption ability of the cartilage, making it increasingly susceptible to further damage. According to the study, changes in the collagen network architecture were a much more important factor affecting cartilage stiffness than the amount of collagen. The ability to protect the collagen network would be an efficient way to slow down the progression of osteoarthritis.
An in-depth understanding of the progression of osteoarthritis can help in the development of conservative prevention strategies and in slowing down the progression of the disease. The results of the study will hopefully facilitate an earlier detection and better treatment of osteoarthritis in the future.
source: University of Eastern Finland