To ensure normal fetal development and prevent disease, it is crucial that certain genes are on or off in the right time intervals. Researchers in Professor Kristian Helin's group at BRIC, University of Copenhagen, have now shown how the TET1 enzyme controls the activity of our genes. The results are just published in the journal Nature.
Control of our genes
The complete human genetic code was mapped in 2000. However, it has become clear that the genetic code itself only in part can answer how an individual develops and is protected against disease. What is detrimental is also how our genes are controlled – what genes are on or off at certain times. This is in part regulated by specific cellular enzymes that can attach small chemical groups, methyl groups, to our DNA:
-The methyl groups can turn off the gene that lies in a stretch of DNA where it is added. TET1 is another type of enzyme that can fine tune the signals that control gene activity by changing the methyl groups which thereafter are removed, says Kristian Helin.
TET1 controls fetal development
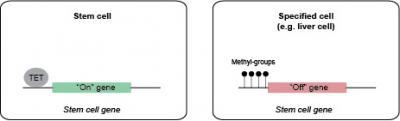
In stem cells the TET1 enzyme turns specialized stem cells genes on by removing methyl groups from the genes. In specialized cells, as for example liver cells, TET1 is not present why methyl groups will turn off the stem cell genes.
(Photo Credit: Kristine Williams, BRIC, University of Copenhagen)
Kristine Williams, Jesper Christensen and Marianne Terndrup Pedersen are the 3 key persons in the Helin laboratory at BRIC contributing with the new results:
-Our most important finding is that TET1 acts like a safe guard and prevents that methyl groups are attached to genes that needs to be active for normal growth and development of our cells. That is crucial for normal fetal development, says PhD student Kristine Williams. Selected genes needs to be active in the stem cells of our body, before the cells are specialized to one of the more than 200 specialized cell types that exist in our body. Other genes need only to be active in specialized cell types as for example liver cells, muscle cells or nerve cells.
When cancer cells develop
The results also contribute to the understanding of what goes wrong when some cells accidently develop into cancer cells. The functions of our body are dependent on constant cellular renewal through division of the cells. A large cellular machinery ensures that our DNA is intact and copied correctly when our cells divide. This is crucial for normal development and function of the cells. In the worst case scenario, changes in the DNA, so called mutations, can result in development of cancer. Specialized genes called tumor suppressor genes are especially important for fighting cancer:
- If methyl groups are deployed to genes that are usually active in normal cells, the genes are turned off and this can be detrimental. If it happens to tumor suppressor genes, it can be a step towards cancer development as the genes no longer can protect against unintended cell growth, says Kristian Helin.
TET enzymes and blood cancers
So TET1 can fight cancers by controlling the activity and protective function of tumor suppressor genes. Our cells also contain a close relative to TET1, the TET2 enzyme, which is the most frequently mutated gene in blood cancers. The researches at BRIC has discovered that TET2 also controls gene activity by facilitating removal of methyl groups from the DNA and they are currently extending these studies to cellular models for cancer development. Results from these studies will supply insight into the mechanisms leading to blood cancers and can potentially lead to development of new therapeutics.
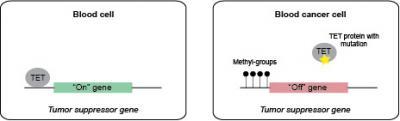
Genes that protect against cancer, so-called tumor suppressor genes can be found in normal blod cells. The TET enzymes turn these genes on by removing methyl groups from the genes. In blod cancer cells, TET2 is often mutated and cannot remove methyl groups from the protective genes, why the genes are shut off.
(Photo Credit: Kristine Williams, BRIC, University of Copenhagen)
Source: University of Copenhagen