WASHINGTON, D.C., March 1, 2016 -- A tiny protein known as an "amyloid beta" acts like Jekyll and Hyde in mysterious ways within the human body. Outsized human suffering is linked to this otherwise tiny, innocuous-looking molecule, as it is suspected to be a key player in the neurodegenerative mechanisms underlying Alzheimer's disease.
The action of any protein, whether it is life-supporting or toxic, is tied to its shape. Amyloid beta molecules appear to become toxic within our bodies when they make contact with each other and form small bundles. Oddly, they may become less toxic again as the bundles grow larger in size and form ordered fibrillary plaque deposits.
This begs the question: What's different about these bundles than the single protein molecule and the fibrils? During the Biophysical Society's 60th Annual Meeting, being held in Los Angeles, Calif., Feb. 27-March 2, 2016, a group of researchers from the Tata Institute of Fundamental Research in India, will describe what they discovered while exploring a suspected critical change in the protein shape as the molecular bundle grows in size.
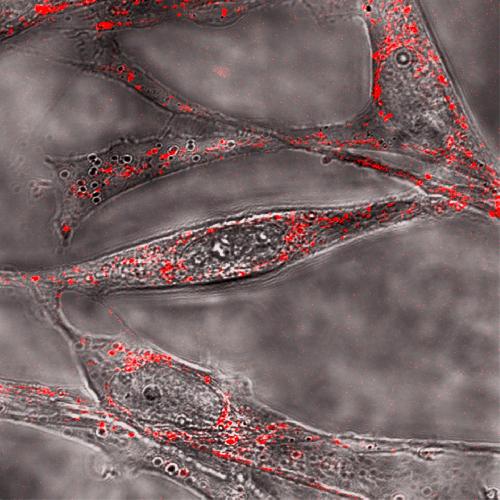 The suspected molecular dagger in Alzheimer's, shown here in red attacking cells (grey), contains a special twist. Credit: Maiti
The suspected molecular dagger in Alzheimer's, shown here in red attacking cells (grey), contains a special twist. Credit: Maiti
"Determining this difference in shape was a challenge, however, because standard tools fail to decipher much about this shape-changing molecule," explained Sudipta Maiti, who co-directed this research. "The power of nuclear magnetic resonance (NMR) couldn't be applied, because the shape change involved is too fast to be captured by it." Faster tools like optical spectroscopy can monitor these fast changes, but don't have high enough resolution to provide detailed information about the molecule's shape.
So the researchers decided to go back to NMR. They "froze" time by literally freezing the structurally evolving sample at "appropriate" times, determined via optical spectroscopic techniques.
"The frozen sample can then be studied at leisure with a variation of NMR called 'solid-state NMR,' as well as other spectroscopy techniques," noted Maiti.
Solid-state NMR "has the power to yield atomic-scale resolution and molecular geometry information in a direct manner," pointed out P.K. Madhu of TIFR Center for Interdisciplinary Sciences, Hyderabad, co-director of this work.
To zero in on underlying changes, they also used high-resolution modeling and simulations, which, according to co-author Ravindra Venkatramani, helped them to visualize the shapes of the molecular components.
One of the biggest questions within this field of study is: How does toxicity evolve? Until now, it's been difficult to explain why toxicity decreases as larger fibrils form.
"By figuring this out, it may be possible to tweak the small bundles so that they take a nontoxic route to forming fibrils," Maiti said. "Our previous research didn't uncover any major differences in the shape of the protein as it evolves from the small bundles to the larger fibrils."
But, with this new approach, they discovered a "twist" within the structure--as the small bundle grows, an intramolecular-to-intermolecular beta sheet transition occurs--that was hidden until now.
"This feature seems to bring the protein closer in shape to some other well-understood proteins with established mechanisms of toxicity," said Maiti. "An intriguing aspect is that this transformation--from toxic to benign--appears to be governed by a sort of 'kiss' between two parts of the protein molecule, an interaction also known as a 'salt bridge.'"
This provides clear structural markers to differentiate between the small and larger forms of the protein, and provides a basis for understanding why the smaller bundles are more toxic.
If the group is correct, their work will open the door to diagnosing the toxic forms of the protein--and possibly even manipulating them. With a greater understanding of the protein's shape, the researchers are now determined to explore how it takes an insidious turn. "Ideally, we'd like to figure out how to manipulate the toxic forms of the protein to make it take the nontoxic path," Maiti said.
For example, "if a potential drug molecule can be synthesized to make the 'kiss' happen at the earliest stage, it may dramatically reduce the toxicity," he added. "We're also excited because our technology can be applied to help understand the molecular basis for other diseases, such as Parkinson's and Type-II diabetes, which are also linked to small proteins."
The group plans to put their theories to the test to see if they work as a general principle, and then "pharmaceutical scientists can pursue it further as a promising avenue for developing drugs to treat many diseases--or even develop a cure," Maiti said.
Presentation #1765, "A hidden structural transition accompanies the progression of amyloid-βeta oligomers to mature fibrils," is authored by Bappaditya Chandra, Debanjan Bhowmik, Barun K. Maity, Debabrata Dhara, Kaustubh Mote, Ravindra Venkatramani, Perunthiruthy K. Madhu and Sudipta Maiti. It will be at 11:00 a.m. PT on Tuesday, March 1, 2016 in Room 515A of the Los Angeles Convention Center. ABSTRACT: http://tinyurl.com/jncs49m
source: Biophysical Society