Ghosh and colleagues found that the density of the M1-fibrinogen structure was a critical characteristic. Looser structures or separate fibers formed by altered versions of M1 failed to trigger a pathological response.
"This research provides the first snapshot of the interaction between this key bacterial virulence factor and its human target at the atomic level," said Victor Nizet, M.D., professor of pediatrics and pharmacy and a co-author of the report.
Difficult to treat once they set in, the leaking blood vessels and organ failure of strep-induced toxic shock prove fatal for 30 percent of patients. Ghosh and Nizet have a long-standing collaboration aimed at designing treatments to counteract the toxic effects of strep protein.
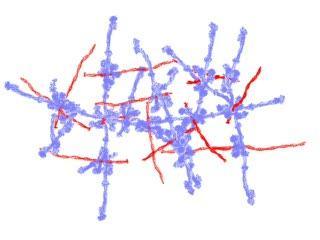
M1 joints (red) and fibrinogen struts (blue) form a scaffold. Dense assemblies trigger a pathological response that can lead to toxic shock.
(Photo Credit: Partho Ghosh lab)
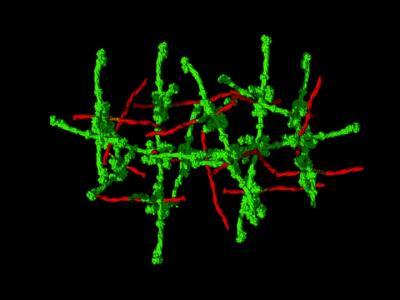
M1 joints (red) and fibrinogen struts (green) form a scaffold. Dense assemblies trigger a pathological response that can lead to toxic shock. Higher resolution image and an additional version with white background available here: http://physicalsciences.ucsd.edu/press/.
(Photo Credit: Partho Ghosh lab/UC San Diego)