Mouse fathers under psychological stress were more likely to have offspring with high blood sugar compared to their unstressed counterparts. In a study appearing February 18 in Cell Metabolism, researchers link this difference to an epigenetic change in the stressed dad's sperm--a change that they could prevent by blocking the father's stress hormones. The study adds to growing evidence that a male's life experience can be passed down through more than his genetic code alone.
"We are very interested in how behavioral change affects glucose homeostasis," says co-senior author Xiaoying Li, an endocrinologist at the Shanghai Jiao Tong University School of Medicine and Rui-Jin Hospital. "Epidemiological studies have demonstrated the association of psychological stress with incident diabetes. We are curious about whether the effect can be passed down through generations."
Li and her colleagues confined male mice in plastic tubes for 2 hr a day, for 2 weeks straight, to induce stress. Afterwards, the animals' glucose levels were increased, but the mice gained weight more slowly and had increased levels of stress hormones called glucocorticoids in their blood. These mice were then mated with females that hadn't been confined, and their resulting offspring had higher blood glucose than normal. "Paternal psychological stress can result in hyperglycemia in offspring in mice," says Li.
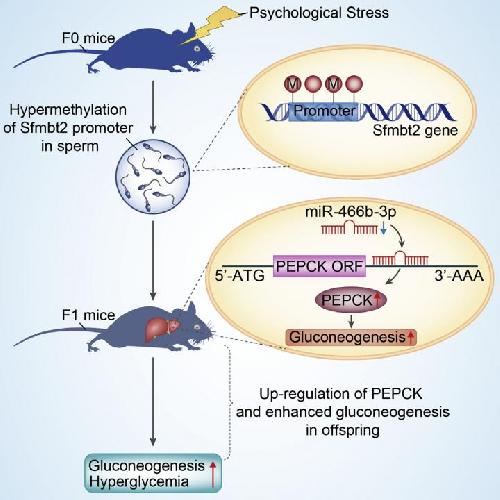 This visual abstract shows how using a mouse model of restraint stress, Wu et al. uncovered the intergenerational effects of paternal psychological stress on glucose metabolism in offspring. Paternal stress epigenetically downregulates miR-466b-3p expression, leading to increased PEPCK expression and hepatic gluconeogenesis in hyperglycemic F1 mice. Credit: Wu, Lu, and Jiao et al./Cell Metabolism 2016
This visual abstract shows how using a mouse model of restraint stress, Wu et al. uncovered the intergenerational effects of paternal psychological stress on glucose metabolism in offspring. Paternal stress epigenetically downregulates miR-466b-3p expression, leading to increased PEPCK expression and hepatic gluconeogenesis in hyperglycemic F1 mice. Credit: Wu, Lu, and Jiao et al./Cell Metabolism 2016
The root of the increased blood sugar was in a gene called Sfmbt2. When a male mouse is immobilized daily in a plastic tube, the spike of glucocorticoids causes extra methyl groups to be added to the Sfmbt2 gene in his sperm. These epigenetic marks don't affect the underlying DNA, but they do control how Sfmbt2 and an associated microRNA (the intronic microRNA-466b-3p) are expressed.
"The epigenetic reprogramming from stress, through glucocorticoids, was surprising," says other senior co-author Xuejin Chen, also at the Shanghai Jiao Tong University School of Medicine.
This epigenetic change in stressed fathers showed up in their offspring's livers. The intronic microRNA-466b-3p in Sfmbt2 is supposed to help regulate an enzyme called PEPCK, which controls sugar production in the liver. But when mammals reproduce, Sfmbt2 is turned off in the egg from the mother--meaning that offspring inherit their only working copy from the father's sperm. And when the only functional Sfmbt2 gene carries these epigenetic tags, the intronic microRNA-466b-3p is silenced and can't keep watch over PEPCK as it normally would. The father's offspring then develop livers with too much PEPCK, causing their blood glucose to increase.
The good news is that by understanding the mechanisms involved, the researchers could block the effects of glucocorticoids on the mouse father's sperm. Injecting the male mice with a molecule that dampens the effect of glucocorticoids stopped the Sfmbt2 gene from being overly methylated. "It is potentially possible for our study to be translated into the treatment of hyperglycemia in human beings in the future," Li says.
source: Cell Press