Research led by the Babraham Institute with collaborators in the UK, Canada and Japan has revealed a new understanding of how an open genome structure supports the long-term and unrestricted developmental potential in embryonic stem cells. This insight provides new avenues for improving the quality and stability of embryonic stem cells - an essential requirement to fulfil their promise in regenerative medicine.
How our DNA is stored and packaged in the nucleus can be viewed as two different states: regions of the genome that are 'open for business' and can be actively read, and regions that are locked away by being tightly packed and inaccessible to the factors that read DNA.
The researchers looked in detail at the mysterious tightly packed portions of the genome, called constitutive heterochromatin. Previous research has shown that heterochromatin is maintained in an unusually open and uncompacted organisation in embryonic stem cells, which is different to all other cell types. It is thought that this rare form of genome architecture may contribute to keeping stem cells in an unspecialised state, still full of the potential to become any cell type in the body. Why heterochromatin is organised in this way in embryonic stem cells has previously been unknown.
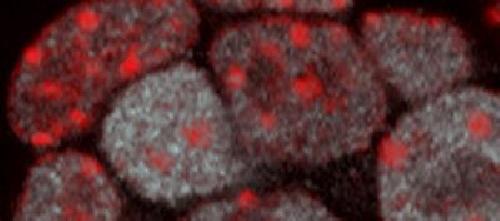 A fluorescent microscopy image of mouse embryonic stem cell nuclei with heterochromatin domains localized in red (using an anti-H3K9me3 antibody) and levels of the stem cell factor NANOG revealed by the intensity of the white signal (using an anti-NANOG antibody). Nuclei with higher NANOG levels tend to have fewer and larger heterochromatin domains, which is one indicator of an open and uncompacted genome organization. Credit: Babraham Institute
A fluorescent microscopy image of mouse embryonic stem cell nuclei with heterochromatin domains localized in red (using an anti-H3K9me3 antibody) and levels of the stem cell factor NANOG revealed by the intensity of the white signal (using an anti-NANOG antibody). Nuclei with higher NANOG levels tend to have fewer and larger heterochromatin domains, which is one indicator of an open and uncompacted genome organization. Credit: Babraham Institute
As described in the journal Genes & Development, the researchers identified a new pathway controlling heterochromatin organisation in mouse embryonic stem cells. Unexpectedly, this pathway assigns new roles for several well-known stem cell factors. The research showed that the stem cell factors, Nanog and Sall1, bind to heterochromatin and help to maintain this portion of the genome in an open form. Embryonic stem cells lacking Nanog and Sall1 showed major defects in heterochromatin organisation, including the closure and compaction of the chromatin. These new findings uncover the first direct connection between stem cell factors and the control of genome architecture, and explains why stem cell heterochromatin is normally in an open and uncompacted form. Loss of heterochromatin regulation has potential consequences for the long-term genetic stability of stem cells, and the ability of stem cells to mature into specialised cell types.
Dr Peter Rugg-Gunn, senior author on the research paper and research group leader at the Babraham Institute explained: "This unanticipated connection between stem cell factors and heterochromatin organisation is important because it tells us about how stem cells work. By tapping into this newly identified connection, we open up new avenues for more successful reprogramming of adult cells to a stem cell state, which is a priority for future regenerative medicine approaches."
source: Babraham Institute