Scientists in China have finally succeeded in creating functioning sperm from mice in the laboratory. To accomplish this feat, the researchers coaxed mouse embryonic stem cells to turn into functional sperm-like cells, which were then injected into egg cells to produce fertile mouse offspring. The work, reported February 25 in Cell Stem Cell, provides a platform for generating sperm cells that could one day be used to treat male infertility in humans.
"Reproducing germ cell development in vitro has remained a central goal in both reproductive biology and reproductive medicine," says co-senior study author Jiahao Sha of Nanjing Medical University. "We established a robust, stepwise approach that recapitulates the formation of functional sperm-like cells in a dish. Our method fully complies with the gold standards recently proposed by a consensus panel of reproductive biologists, so we think that it holds tremendous promise for treating male infertility."
Infertility affects up to 15% of couples, and about one-third of cases can be traced to the man. One major cause of male infertility is the failure of precursor germ cells in the testes to undergo a type of cell division called meiosis to form functional sperm cells. Several studies have reported the successful generation of germ cells from stem cells, but they did not fully evaluate the functionality of the germ cells or provide proof for all key hallmarks of meiosis.
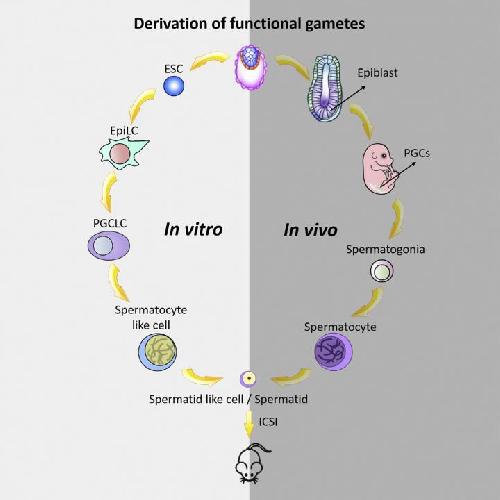 This graphical abstract shows how Zhou et al. generated haploid male gametes from mouse embryonic stem cells that can produce viable and fertile offspring, demonstrating functional reproduction of meiosis in vitro. Credit: Zhou, Wang, and Yuan et al./Cell Stem Cell 2016
This graphical abstract shows how Zhou et al. generated haploid male gametes from mouse embryonic stem cells that can produce viable and fertile offspring, demonstrating functional reproduction of meiosis in vitro. Credit: Zhou, Wang, and Yuan et al./Cell Stem Cell 2016
Recently, a panel of reproductive biologists proposed gold standard criteria to prove that the major events of meiosis have taken place in engineered germ cells. For example, researchers must show evidence of the correct nuclear DNA content at specific meiotic stages, normal chromosome number and organization, and the capacity of germ cells to produce viable offspring. Until now, the recapitulation of all of the essential steps of meiosis has remained a major obstacle to the production of functional sperm and egg cells in a dish.
To overcome this hurdle, Sha teamed up with co-senior study authors Qi Zhou and Xiao-Yang Zhao of the Institute of Zoology at the Chinese Academy of Sciences to develop a stem cell-based method that fully recapitulates meiosis and produces functional sperm-like cells. The first step was to expose mouse embryonic stem cells (ESCs) to a chemical cocktail, which coaxed the ESCs to turn into primordial germ cells. Next, the researchers mimicked the natural tissue environment of these precursor germ cells by exposing them to testicular cells as well as sex hormones such as testosterone.
Under these biologically relevant conditions, the ESC-derived primordial germ cells underwent complete meiosis, resulting in sperm-like cells with correct nuclear DNA and chromosomal content. To provide final gold-standard proof of meiosis, the researchers injected these sperm-like cells into mouse egg cells and transferred the embryos into female mice. Remarkably, these embryos developed normally and gave rise to healthy, fertile offspring, which gave birth to the next generation.
In future studies, the researchers plan to use their platform to examine the molecular mechanisms controlling meiosis. They will also test their approach in other animals such as primates in anticipation of human studies. However, before this technique is translated to the clinic, possible risks must be ruled out and ethical concerns regarding the use of embryonic cells should be considered.
"If proven to be safe and effective in humans, our platform could potentially generate fully functional sperm for artificial insemination or in vitro fertilization techniques," Sha says. "Because currently available treatments do not work for many couples, we hope that our approach could substantially improve success rates for male infertility."
source: Cell Press