This Special Issue on the microbiome features three reports, three Reviews and a Perspective that capture the many ways in which the microbes within our gut influence our health, and what in turn shapes this complex community.
First, data on gut microbes from perhaps the two largest cohorts to date provides an unprecedented view into the microbiome. Gwen Falony et al. analyzed fecal samples, physiological measurements and survey data from the Belgian Flemish Gut Flora Project and the Dutch LifeLines-Deep study, as well as several other global datasets, which collectively represent nearly 4,000 people. Their analysis identified 14 core gut microbes that are found in more than 95% of people, and 664 species in total. Out of 503 different parameters, stool consistency was the strongest variable associated with microbiome composition. As well, the researchers were able to draw associations between genus abundances and hip circumference, uric acid concentrations, amoxicillin intake, and even chocolate-type preference. The most significant factor behind microbiome variation in this study was the use of drugs, including antibiotics, osmotic laxatives, inflammatory bowel disease medication, female hormones, benzodiazepines, antidepressants, and antihistamine. Also, some early-life events generally thought to affect adult microbiota composition - including mode of birth (cesarean section or vaginal delivery), and infant nutrition (breastfed or not) - were not associated with microbiota composition variation. Analysis by Falony et al. confirms (or, at least, does not discount some evidence) that certain microbes are associated with diseases (i.e., ulcerative colitis and colorectal cancer). Regarding the ongoing debate around the association between microbiome composition and body mass index (BMI), the researchers' analyses revealed that effect size is small, but significant. Collectively, these data provide numerous and important insights into microbiome variation across individuals.
In a second study, Alexandra Zhernakova et al. analyze data from 1,135 participants in the LifeLines-DEEP cohort, finding correlations between a person's health and diet, and certain microbe species. While they identified a number of secreted proteins that could act as biomarkers of microbiota health, chromogranin A (CgA) showed the strongest association. Their analysis also reveals many dietary correlations; for example, features of a Western-style diet, such as higher intake of total energy, snacking, and high-fat milk, were associated with lower microbiota diversity, as well as a higher amount of carbohydrates. Consumption of sugar-sweetened soda had a negative effect on microbial diversity, whereas consumption of coffee, tea, and red wine was associated with increased diversity. Results from this study confirm some previous studies that find that antibiotic use causes a decline in certain species. Several other drug categories - metformin, statins, laxatives and particularly proton-pump inhibitors- also had a strong effect on the gut microbiome. Remarkably, even heart attacks may change the microbiome, as individuals in this study who had suffered a heart attack had a significantly lower abundance of Eubacterium eligens bacterium.
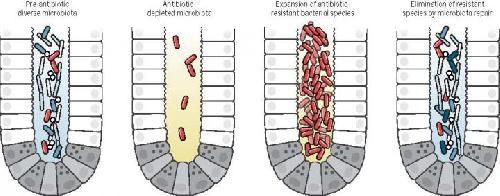 Antibiotic treatment eliminates many commensal bacterial species from the gut lumen and reduces antimicrobial defenses. This material relates to a paper that appeared in the April 29, issue of Science, published by AAAS. The paper, by E.G. Pamer at Memorial Sloan Kettering Cancer Center in New York, NY, and colleagues was titled, "Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens." Credit: Pamer et al. / Science (2016)
Antibiotic treatment eliminates many commensal bacterial species from the gut lumen and reduces antimicrobial defenses. This material relates to a paper that appeared in the April 29, issue of Science, published by AAAS. The paper, by E.G. Pamer at Memorial Sloan Kettering Cancer Center in New York, NY, and colleagues was titled, "Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens." Credit: Pamer et al. / Science (2016)
A third study by Simone Li et al. sheds light on the mysterious microbial dynamics that occur following fecal transplant, identifying species that are particularly good at colonizing a recipient's gut, and that can persist for significant duration following transplant. Fecal transplants, where stool from a healthy donor is transplanted into the gut of another, have been shown to be beneficial for a range of diseases and particularly for treating Clostridium difficile infection. However, the microbiota dynamics following fecal transplant are largely unknown and many previous studies involved pre-treatment with antibiotics, which disturb the gut microbiota. As well, it is difficult to distinguish between donor and recipient strains of microbes. Thus, Li et al. analyzed stool samples of 10 recipients who were not treated with antibiotics, before and after fecal transplants. To monitor specific donor and recipient strains of microbes, the researchers looked for unique genetic variants of each one. Their data suggest that a significant portion of donor-specific strains were still present up to three months later. They identified three species that were exceptionally good at colonizing the recipient's gut.
In a Review, Eric Pamer discusses potential therapies targeting the microbiota that may be important for fighting antibiotic-resistant infections. Antibiotic treatment can damage the microbiota and, paradoxically, allow certain microbes that are resistant to antibiotics to flourish, thus increasing a person's susceptibility to infections. Re-establishing "good" microbes could help counter this problem. Pamer highlights several types of beneficial microbes that have been identified, but notes issues surrounding the commercial development and patenting of bacterial species.
Although the human microbiome is predominantly influenced by diet and medication, a review by Julia Goodrich and colleagues highlights several studies demonstrating that certain types of microbes that live in the gut are at least partly controlled by human genetics. For example, a handful of Bacteria and Archaea, with known importance for health, are associated with host genes related to immunity and diet.
In a third review, Thomas Gensollen and colleagues summarize studies to date that identify ways in which the microbiota influences the mammalian immune system during infancy, sometimes with lasting implications through adulthood. For example germ-free mice tend to have higher levels of invariant natural killer T (iNKT) in their lungs, which increases their susceptibility to colitis - this can be treated by colonizing the germ-free mice with certain microbes during the first two weeks of life, but not thereafter. These studies in germ-free mice, in combination with familial studies, demonstrate how the microbiota and a host's immune system are intricately connected. Lastly, in a Perspective, Martin Blaser discusses the consequences of antibiotic use for the healthy microbiota. Those most sensitive to microbiota disturbance, young children, are the most likely to be treated with antibiotics. He proposes several ways to potentially restore a healthy microbiota following treatment with antibiotics. In the meantime, he says, rather than "carpet-bombing germs into submission with broad-spectrum antibiotics," we need to develop more targeted therapies against specific pathogens, minimize damage to essential symbiotic microbial species, and preserve community structure and function in the healthy microbiome.
source: American Association for the Advancement of Science