Baltimore, MD--New work from Carnegie's Allan Spradling and Lei Lei demonstrates that mammalian egg cells gain crucial cellular components at an early stage from their undifferentiated sister cells, called germ cells. This mechanism had previously only been documented in lower animals, and may be a key to understanding the egg's unique properties. Their work is published via Science First Release.
Egg cells are the only cells in humans and other animals that are capable of developing into a new individual. Yet only a small number of egg cells are produced by the female body, so they are the limiting factor in many aspects of reproductive science. Understanding how eggs gain the power to develop is crucial to basic scientists, as well as to doctors interested in improving a female's reproductive odds by increasing their egg supply.
Working with mice, Lei and Spradling set out to test their belief that certain undifferentiated germ cells learn to develop into eggs very early during their production in the ovary, when germ cells are found in small clusters of interconnected sister cells, all daughters of the same parent cell.
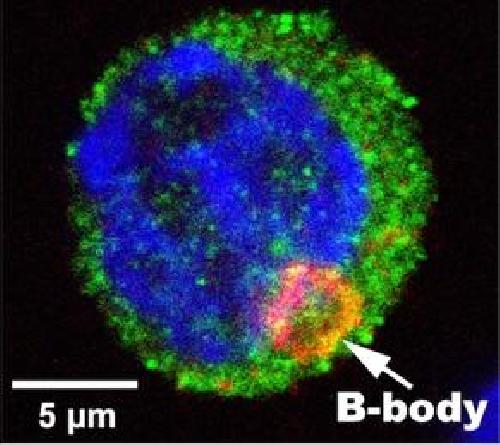 This is a mouse egg cell after transfers from nurse cells are complete. Note the nucleus (DNA: blue), cytoplasm (germ cell marker: green) and the Balbiani body ("B-Body"; Golgi marker: orange). Credit: Allan Spradling and Lei Lei
This is a mouse egg cell after transfers from nurse cells are complete. Note the nucleus (DNA: blue), cytoplasm (germ cell marker: green) and the Balbiani body ("B-Body"; Golgi marker: orange). Credit: Allan Spradling and Lei Lei
Similar clusters have been observed in lower animals such as the fruit fly. In flies, most of the germ cells, known as nurse cells, transfer their cellular organs, called organelles, and cytoplasm, the fluid solution in which the organelles are suspended, to just one cell, which then becomes an egg.
However, until now, the germ cell clusters in mammalian ovaries were thought by most researchers to be a non-functional, evolutionary relic. But Lei and Spradling found that as in fruit flies, these connected, mammalian sister germ cells also exchange cytoplasm and organelles--including mitochondria, the cell's energy factory; Golgi, the cell's packaging service; and centrosomes, which manage cell division.
Among the five germ cells in an average mouse cluster, four cells transfer most of their organelles into the fifth, which alone becomes an egg cell. The new organelles coalesce into a structure called a Balbiani body, a dark, circular blob seen only in young eggs but whose origin was previously unknown.
When the researchers used lab techniques to block this transfer, few egg cells were able to form. So they were able to demonstrate that like insects and invertebrates, mammals undergo an evolutionarily conserved process of organelle transfer from the nurse cells to the future egg that is likely to be functionally important.
How might this sacrifice by the nurse cells help the egg cell gain the power to develop?
The acquisition of so much cellular material from its sisters almost certainly helps the egg start a program of growth through which it becomes the largest cell in the mammalian body. Lei and Spradling also hypothesize that the transfer includes additional factors that "reprogram" the egg's chromosomes and give it the capacity to develop into an embryo. The Balbiani body, itself generated by the transfer, might further support egg development by producing nutrients or hormones. Finally, damaged cellular organelles might be moved outward during transfer, away from the egg, purifying it.
"Understanding how mammalian oocytes are built will greatly assist efforts to combat human infertility and to improve animal husbandry," Lei commented.
"This work also shows the importance of using model organisms such as the fruit fly Drosophila to study subjects of medical importance," Spradling added. "Research on Drosophila guided this work, and will be essential to identifying the specific human factors and their genes that re-program egg cells and make development possible."
source: Carnegie Institution