As a result of mapping the structure of the protein complex implicated in autism spectrum disorders, a research team led by scientists at University of California, San Diego have discovered how particular genetic mutations affect this complex and contribute to the developmental abnormalities found in children with autism. Their work should help scientists pinpoint the consequences of other genetic abnormalities associated with the disorder.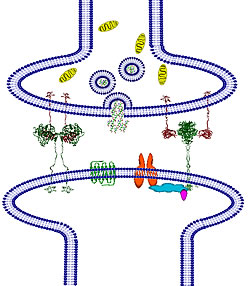 Neuroligin/neurexin complex in the synapse
Neuroligin/neurexin complex in the synapse
“By understanding the three-dimensional structure of the normal protein, researchers can now make predictions about how mutations in the gene affect the structure of the gene product,” said first author Davide Comoletti, Ph.D., UCSD research associate at the Skaggs School of Pharmacy.
Autism spectrum disorders are developmental disabilities that cause impairments in social interaction and communication. Both children and adults with autism typically show difficulties in verbal and non-verbal communication, interpersonal relationships, and leisure or play activities.
Comoletti and colleagues studied the neuroligin family of proteins that are encoded by genes known to be mutated in certain patients with autism. The neuroligins, and their partner proteins, the neurexins, are involved in the junctions, or synapses, through which cells of the nervous system signal to one another and to non-neuronal tissues such as muscle. These structural studies on neuroligins and neurexins represent a major step toward defining the synaptic organization at the molecular level.
“Normally, individual neuroligins are encoded to interact with specific neurexin partners. The two partners are members of distinct families of proteins involved in synaptic adhesions, imparting ‘stickiness’ that enables them to associate so that synapses form and have the capacity for neurotransmission,” said Palmer Taylor, Ph.D., Dean of the Skaggs School, Sandra & Monroe Trout Professor of Pharmacology, and co-principal investigator of the study, along with Jill Trewhella, Ph.D., of the University of Sydney, Australia and University of Utah.
Incorrect partnering that results when a mutant neuroligin fails to properly align at synapses helps explain why some autism spectrum disorders are manifested in subtle behavioral abnormalities that are seen at an early age.
“Abnormal synaptic development in nerve connections is likely to lead to cognitive deficits seen in patients with autism,” said Taylor. He added that synapse formation and maintenance occurs early in development when the infant brain is still plastic and formative. Therefore, by understanding the structural mutations that affect neurotransmission during development, new leads into drug therapies may emerge.
“We really don’t know what causes autism, but this research represents a solid starting point,” said Sarah Dunsmore, Ph.D., program director with the National Institute of General Medical Sciences, part of the National Institutes of Health, which partly supported the study. “The work suggests that genetic mutations that alter the shape or folding of adhesion proteins in the nervous system influence their interactions. This is another example of how research on basic biological questions, such as the three-dimensional structures of proteins in the brain, can yield valuable medical insights.”
Taylor and colleagues have been studying the structure and function of acetylcholinesterase – a structurally related protein that mediates neurotransmission between nerves and between nerve and muscle – for the past 30 years. They began studying the neuroligins because of the similarity in structure and amino acid sequence with acetylcholinesterase.
The study was a multi-national collaboration, employing a synchrotron and a neutron source at two national laboratories to collect the X-ray and neutron scattering data necessary for resolving the structure. Additional contributors to this study include Alexander Grishaev, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; Andrew E. Whitten, Bragg Institute, Australian Nuclear Science and Technology Organization; and Igor Tsigelny, UCSD Department of Pharmacology, Skaggs School of Pharmacy and Pharmaceutical Sciences.This work was supported in part by grants from the National Institutes of Health, the U.S. Department of Energy, and the Cure Autism Now Foundation.
Source: UCSD