Such cluster "hopping" between two positions is known as two-state dynamics, a signature property of glass. In a glass, the atoms or molecules are randomly positioned or oriented, much the way they are in a liquid or gas. But while atoms have much more freedom of motion to diffuse through a liquid or gas, in a glass the molecules or atom clusters are stuck most of the time in the solid. Instead, a cluster usually has only two adjoining places that it can ferry between.
"This is the first time that this type of two-state hopping has been imaged in a-Si," Gruebele said. "It's been predicted by theory and people have inferred it indirectly from other measurements, but this is the first time we're been able to visualize it."
The group's observations of two-state dynamics show that pure a-Si is indeed a glass, in spite of its unorthodox manufacturing method. However, a-Si is rarely used in its pure form; hydrogen is added to make it more stable and improve performance.
Researchers have long assumed that hydrogenation has little to no effect on the random structure of a-Si, but the group's observations show that this assumption is not quite correct. In fact, adding hydrogen robs a-Si of its two-state dynamics and its categorization as a glass. Furthermore, the surface is riddled with signs of crystallization: larger clusters, cracks and highly structured patches.
Such micro-crystalline structure has great implications for the properties of a-Si and how they are studied and applied. Since most research has been conducted on hydrogenated a-Si, Gruebele sees a great opportunity to delve into the largely unknown characteristics of the glassy state.
"In some ways, I think we actually know less about the properties of glassy silicon than we think we do, because a lot of what's been investigated of what people call amorphous or glassy silicon isn't really completely amorphous," Gruebele said. "We really need to revisit what the properties of a-Si are. There could yet be surprises in the way it functions and the kind of things that we might be able to do with it."
Next, the group hopes to conduct temperature-depended studies to further establish the activation barriers, or the energy "humps" that the clusters must overcome to move between positions.
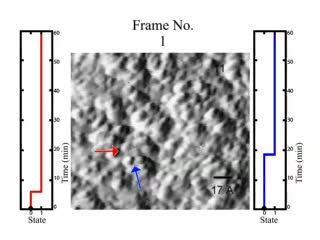
A time-lapse video of an amorphous silicon surface. The lumps are clusters of about five atoms of silicon. The "hopping" motion of the lumps shows that a-Si is a glass.
(Photo Credit: Martin Gruebele)
University of Illinois chemistry professor Martin Gruebele led a group that directly observed two-state dynamics in glassy silicon for the first time.
(Photo Credit: L. Brian Stauffer)