Generating fully functional pancreatic beta cells in the lab has been a challenge for diabetes researchers. When human stem cells develop into beta cells in a dish, they only reach a precursor stage, unable to fully mature; this prevents them from effectively producing insulin in response to glucose. Now, scientists reporting April 12 in Cell Metabolism have discovered a protein that activates the maturation process in vitro, overcoming this longstanding obstacle in diabetes therapy development.
"In a dish, with this one switch, it's possible to produce a functional human beta cell that's responding almost as well as the natural thing," says senior author Ronald Evans, a molecular biologist at the Salk Institute. "This has been a major blockade, and overcoming it has been a major challenge to the field."
To create different cell types in the lab, stem cells must be coaxed down the road of determination--the branching paths that fetal cells normally travel to become neurons, skin cells, muscle cells, or any number of other cell types. But there are many developmental points between a stem cell and a fully grown cell type, and for pancreatic beta cells, the stem cells have historically stalled out in an early stage when grown in the lab. "Everyone got stuck at this point," says Evans.
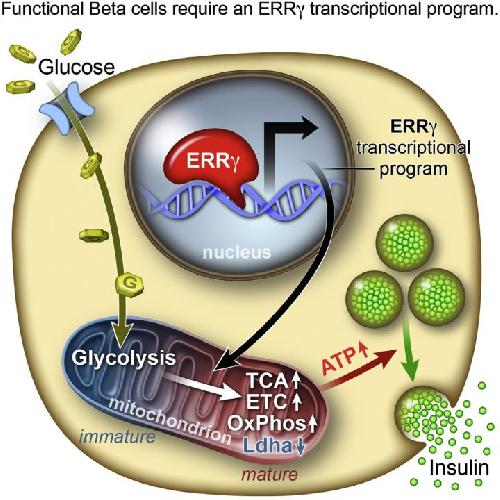 This visual abstract depicts the Yoshihara et al. report that the postnatal maturation of pancreatic b cells necessary for maximal glucosestimulated insulin secretion is coordinated by the estrogen-related receptor g (ERRg). ERRg drives a transcriptional program promoting mitochondrial oxidative metabolism, and its expression in iPSC-derived b-like cells generates functional b cells in vitro. Credit: Yoshihara et al./Cell Metabolism 2016
This visual abstract depicts the Yoshihara et al. report that the postnatal maturation of pancreatic b cells necessary for maximal glucosestimulated insulin secretion is coordinated by the estrogen-related receptor g (ERRg). ERRg drives a transcriptional program promoting mitochondrial oxidative metabolism, and its expression in iPSC-derived b-like cells generates functional b cells in vitro. Credit: Yoshihara et al./Cell Metabolism 2016
To nail down the differences between fetal and adult beta cells and determine what might trigger the next step in the process, Evans and his colleagues analyzed human cells' transcriptomes. They discovered that a nuclear receptor protein, estrogen-related receptor γ (ERRγ), occurred in much larger amounts in adult beta cells. Evans' team had previously worked with the protein, which helps to promote endurance running.
"In muscle, ERRγ induces greater mitochondrial growth and promotes oxidative use of sugars and lipids to generate energy," says Evans. "It was a little bit of a surprise to see that beta cells produce a high level of this regulator, but beta cells have to release massive amounts of insulin quickly to control sugar levels. It's a very energy-intensive process."
When the researchers raised mice that lacked ERRγ, the animals' beta cells couldn't produce insulin in response to blood glucose spikes. But when the team instructed human beta-like cells grown in the lab to produce more ERRγ, "Voilà," says Evans. "Those cells in culture began to respond to glucose and release insulin."
To further test the dish-matured cells, the researchers transplanted them into diabetic mice. From the first day of transplantation, the cells produced insulin in response to glucose spikes in the mice's blood, alleviating the modeled diabetes. "We were very excited when we saw that," says Evans. Turning on the ERRγ switch alone is sufficient to mature the beta-like cells in vitro, with the power to rescue diabetes in vivo, the team concludes.
Looking back, under constant lab conditions, says Evans, grown beta cells were simply stuck in the fetal stage. When a fetus is developing, it receives steady levels of glucose from the mother and doesn't have to produce insulin to control its blood sugar. "We think this molecular ERRγ switch is a critical event to achieving the adult functionality," says Evans, adding that the switch is likely flipped normally when an infant takes its first breath, which oxygenates the blood and helps trigger oxidative metabolism.
"I believe this work transitions us to a new era in creating functional beta cells at will," says Evans. The researchers are planning to explore this process in more complicated models for treating diabetes.
source: Cell Press