Modifying a protein from a plant much favored by science, researchers at the University of California, San Diego School of Medicine and colleagues have created a new type of genetic tag visible under an electron microscope, illuminating life in never-before-seen detail.
Led by Nobel laureate Roger Tsien, PhD, Howard Hughes Medical Institute investigator and UCSD professor of pharmacology, chemistry and biochemistry, a team of scientists radically re-engineered a light-absorbing protein from the cress plant Arabidopsis thaliana. When exposed to blue light, the altered protein produces abundant singlet oxygen, a form of molecular oxygen that can be made visible by electron microscopy (EM).
The work is published in the April 5 issue of the journal Public Library of Science (PLoS) Biology. Tsien was co-winner of the 2008 Nobel Prize in chemistry for his role in helping develop and expand the use of green fluorescent proteins (GFP), a tool widely employed in light microscopy to peer inside living cells or whole animals and observe molecules interacting in real-time.
Tsien said the development of the small, highly engineered Arabidopsis protein, dubbed "miniSOG," may elevate the abilities of electron microscopy in the same way GFPs have made modern light microscopy in biological research much more powerful and useful.
"The big advantage of EM is that it has always had much higher spatial resolution than light microscopy. You can get up to a hundred-fold higher useful magnification from EM than from light microscopy," said Tsien. The result has been extraordinarily detailed, three-dimensional images of microscopic objects at resolutions measuring in the tens of nanometers, tiny enough to meticulously render the internal anatomy of individual cells.
But current EM technologies do not distinguish or highlight individual proteins in these images. These can be tagged with GFP or other fluorescent proteins, but they are visible only with the limited resolution of light microscopy.
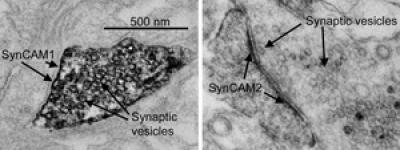
The new electron microscopy technique reveals the previously unknown locations of two neuronal proteins called SynCAM1 and SynCAM2. The first is an adhesion protein found at the synapse -- or communications link -- of neurons sending information. Its close relative, SynCAM2, is used by neurons receiving information. Neurons that send information are distinguishable because they contain synaptic vesicles, which are used to store neurotransmitters for communications use. In these images, the vesicles resemble small hollow circles.
(Photo Credit: UC San Diego School of Medicine)
"Existing EM images of cell structures are analogous to maps or aerial photographs showing major landmarks and populations centers," Tsien said. "It was very difficult to see where individual protein types were located, just as most geographical maps do not show the location of individual classes of people, such as everyone with the surname Smith, or all of the orthodontists in an area. Our new technique enables us to put beacons on just about any protein and get a snapshot of its location at the much higher resolution of EM."
To create this ability, the scientists began with a protein from Arabidopsis, a small flowering plant that has long used as a research model. The original protein absorbs incoming blue light, triggering biochemical signals that inform the plant how much sunlight it is receiving. "We rationally engineered the protein based on its atomic model so that it changes incoming blue light into a little bit of green fluorescence and a lot of singlet oxygen," said the paper's first author, Xiaokun Shu, now an assistant professor at UC San Francisco.
Established methods were then used to convert singlet oxygen production into a tissue stain that the electron microscope can see. The scientists tested the modified protein's utility as an EM marker by first using it to confirm the locations of several already well-understood proteins in mammalian cells, nematodes and rodents, then used miniSOG to successfully tag two neuronal proteins in mice whose locations had not been known.
Tsien and Shu are optimistic that miniSOG will grant new powers to electron microscopy, permitting scientists to pursue answers to questions previously impossible to ask.
"MiniSOG would be much appreciated by scientists who investigate cellular and subcellular structures including neuronal circuits at nanometer resolution in multicellular organisms," said Shu, "because previous methods have great difficulty in achieving both efficient labeling and good preservation of the structures under study."
EM will not replace light microscopy.
"When we use miniSOG, we see the tagged proteins plus the landmarks that we are used to navigating by," said Tsien. "On the other hand, EM has the disadvantage that it gives a snapshot of cells before we killed them (to make the image), whereas light microscopy can show the dynamics in live cells. Each technique has different complementary strengths and weaknesses."

This is Nobel laureate Roger Tsien, Ph.D.
(Photo Credit: UC San Diego School of Medicine)