An unusual regulatory mechanism that controls the swimmer/non-swimmer option in genetically identical Salmonella also impacts the bacteria's ability to cause infection.
University of Washington scientists reported the discovery this week in the Proceedings of the National Academy of Sciences.
As Salmonella divides into genetically identical clones, either of the two forms of the bacteria can emerge. Some individuals sport flagella – thin, whip-like projections that propel the bacterium. Others do not.
When grown in a lab dish, both types appear.
Salmonella is a common food-poisoning bacteria. It can survive and take hold in hostile environments – like inside of people who are protected by hoards of infection-fighting cells. In immunocompromised individuals, it causes blood stream infections.
"In an unpredictable world," said Dr. Brad Cookson, professor of microbiology and laboratory medicine, and division head of Clinical Microbiology at the UW, "Salmonella have evolved to hedge their bets." Each guise has its own advantages and drawbacks in invading a host and evading defenses, depending on the situation.
Cookson explained that being genetically the same but differing in appearance and function is useful for a population of disease-causing bacteria. Even though the bacteria might be identical clones, varying characteristics of the individuals – some of whom are mobile and some of whom are stationary -- allows the population to colonize a host and establish an infection.
"Diversity," Cookson noted, "improves the chance that some of the clones will thrive in fluctuating environments." To infect an animal, swallowed Salmonella breaches the protective mucous of the gut, colonizes the lymph tissues, and then builds a niche inside germ-killing cells. These cells are co-opted into ferrying the Salmonella to the spleen and other organ system tissues.
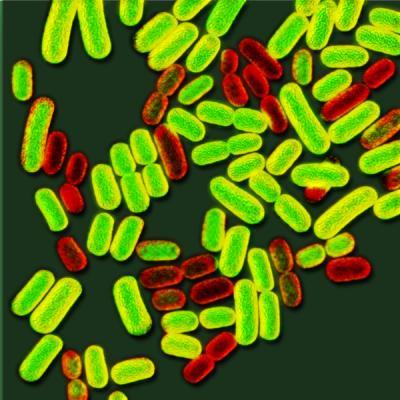
In this magnified image of genetically identical Salmonella, the bacteria shown in yellow-green are manufacturing the protein needed to build flagella to swim, and the reddish ones are not.
(Photo Credit: Kate Sweeney/UW)
"The ability to swim," Cookson said, "is presumably a critical survival trait." In an intestinal infection, for example, motile Salmonella bacteria grow faster than their non-motile counterparts because they are able to migrate to the nutrient-rich layers of the intestinal lining. Inside of macrophages, however, non-motile Salmonella have the upper hand.
That is because the protein needed to make flagella, called flagellin, provokes the body's defenses. When Salmonella bacteria secrete flagellin, their macrophage ferry interprets this as a danger signal, and kills itself -- and the Salmonella on board -- in a self-destructive, pro-inflammatory response called pyroptosis: to go up in flames.
As a countermeasure, Salmonella restricts the production of flagellin to avoid tripping the macrophage alarm. This helps Salmonella avoid inciting an inflammatory response that would lessen its chances of colonizing its host.
Cookson's team of researchers, led by Dr. Mary Stewart from the UW Department of Microbiology, identified the genetic regulation for the "ON" and "OFF" production of flagellin. This genetic regulation pathway is behind the uncanny ability of genetically identical Salmonella to generate physically distinct subgroups.
They discovered that a protein that is almost in a class by itself --YdiV -- determines whether a Salmonella cell will produce or not produce flagellin. This protein can prevent certain parts of the Salmonella genome from being read to manufacture a substance called a sigma factor. The sigma factor plays a key role in recruiting other biochemical functions that promote the production of flagellin.
The sigma factor is repressed in only some of the genetically identical cells. This results in the two kinds of subpopulations: those cells that produce flagellin and those that don't. In a lab dish, both types maintain a stable presence within the Salmonella clonal population. In an animal or human, anatomical location determines which type will fare better during each stage of the infection.
The researchers also found that Salmonella strains that lack the YdiV protein are unable to fully repress the production of flagellin. These mutant strains are less infectious. Looking at the other side of the host-pathogen struggle, mutant mice that couldn't launch pyroptosis in response to flagellin were more susceptible to serious Salmonella infections.
Source: University of Washington