Scientists have designed a molecule which, in living cells, emits turquoise light three times brighter than possible until recently. This improves the sensitivity of cellular imaging, a technique where biological processes inside a living organism are imaged at high resolution. The results have been published in Nature Communications on 20 March 2012.
The lead author of the publication is Antoine Royant from the Institut de Biologie Structurale (CNRS/CEA/University Joseph-Fourier) in Grenoble. The team also comprised scientists from the Universities of Amsterdam and Oxford and from the European Synchrotron Radiation Facility (ESRF) in Grenoble.
Cyan fluorescent proteins (CFPs) are very popular in cell biology where they are used to make visible, like in a movie, processes inside a living cell or changes in the shape of large biological molecules. Since the early 1990s, fluorescent proteins have become one of the most important tools used in the biosciences and have helped the observation of previously invisible processes such as the development of nerve cells in the brain or how cancer cells spread. The 2008 Nobel Prize in Chemistry crowned their discovery and rapid development.

This image is an artistically inspired visualization of the three-dimensional X-ray structure of the Cyan Fluorescence Protein mTurquoise2.
(Photo Credit: Nature Communications/von Stetten/Royant/Goedhart)
CFPs allow mapping of many processes in living cells when they can be attached to a protein involved in an interaction or a conformational change. The CFP inside the cell, and thus the target of the observation, is localised by illuminating the cell with blue light which makes the fluorescent protein emit light of a characteristic colour, which is cyan for CFPs. However, these molecules have long suffered from a weak fluorescence level, converting merely 36% of the incoming blue into cyan light.
To achieve higher brightness, and with it improved sensitivity of fluorescent imaging, the scientists based in France, led by Antoine Royant, teamed up with colleagues from the Netherlands and the United Kingdom.
First, using highly brilliant X-ray beams at the ESRF, the teams from Grenoble and Oxford uncovered subtle details of how CFPs store incoming energy and retransmit it as fluorescent light: they produced tiny crystals of many different improved CFPs and resolved their molecular structures. These structures revealed a subtle process near the so-called chromophore, the light-emitting complex inside the CFPs, whose fluorescence efficiency could be modulated by the environment. "We could understand the function of individual atoms within CFPs and pinpoint the part of the molecule that needed to be modified to increase the fluorescence yield" says David von Stetten from the ESRF.
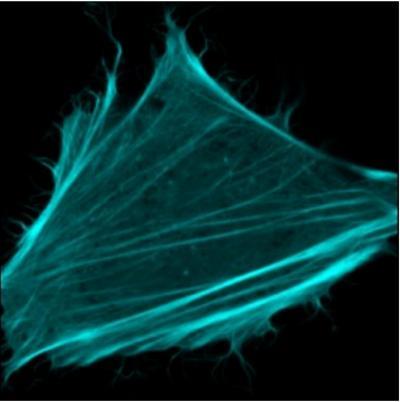
This is an image of the actin filaments in a living cell that was taken using a fluorescence microscope. These filaments are major drivers for example of muscle contraction, and the fluorescing filaments can make this vital process visible in a living cell. Here, mTurquoise2 proteins were fused to a small protein that attaches itself to the actin filaments, allowing these to be visualized.
(Photo Credit: Nature Communications/Goedhart)
In parallel to this work, the Amsterdam team led by Theodorus Gadella used an innovative screening technique to study hundreds of modified CFP molecules, measuring their fluorescence lifetimes under the microscope to identify which had improved properties.
The result of this rational design is a new CFP, called mTurquoise2. By combining structural and cellular biology efforts, the researchers managed to show that mTurquoise2 has a fluorescence efficiency of 93%, unmatched for this type of proteins.
The new molecule will allow life scientists to study protein-protein interactions in living cells with unprecedented sensitivity. High sensitivity matters in processes where only a few proteins are involved and signals are weak, and in fast reactions where the time available for accumulating fluorescent light is short.
"With the new protein, many studies can now be performed with levels of accuracy and detail that were impossible yesterday. Moreover, thanks to this novel approach taking into account the structural dynamics of the protein, scientists now hope to design improved fluorescent proteins emitting light of different colours for use in other applications" concludes Antoine Royant.

This is a photo taken with a microscope of one of the tiny crystals of mTurquoise2 molecules used in this study to understand the interactions, at the atomic scale, that result in the high fluorescence efficiency of mTurquoise2. The scale bars represent 0.1 mm.
(Photo Credit: von Stetten/Royant/ESRF/CNRS)