Sodium, such that is found in ordinary table salt, is constantly transported back and forth our cells membrane in exchange for protons. This regulates sodium levels, cell volume and internal pH. Researchers have now been able to show the details in how the protein NapA carries out this process. In 2013, the researchers reported that the sodium-proton transport process in NapA involved large movements in the protein (http://www.nature.com/nature/journal/v501/n7468/full/nature12484.html#videos), which was surprising because the small size of the molecules transported. However, this model did not have a detailed picture of every step of this transport process.
"Through some neat biochemistry we have now been able to verify these movements and show this complete process by determining one of the missing steps. Intriguingly, just as we originally predicted, this protein moves just like an "elevator" moving the sodium up and down across our cell's membrane." says Dr. David Drew, researcher at the Department of Biochemistry and Biophysics at Stockholm University.
Understanding the fundamental processes is an important step for basic science and could have important implications for health problems related to cancer and hypertension.
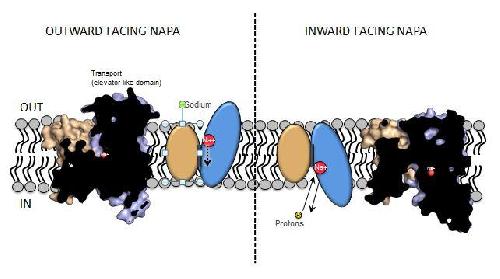 This is a model of NapA. Credit: David Drew
This is a model of NapA. Credit: David Drew
The technique used for determining the structure of the sodium/proton transporter is called x-ray crystallography. Through analysis of the positions of the atoms, researchers have managed to build three-dimensional models of NapA. The article "Crystal structures reveal the molecular basis of ion translocation in sodium/proton antiporters" is published in the scientific journal Nature Structure and Molecular Biology on 1 February 2016.
source: Stockholm University