PHILADELPHIA - Calpain, a calcium-regulated enzyme, is essential to a host of cellular processes, but can cause severe problems in its overactivated state. It has been implicated as a factor in muscular dystrophy, AIDS, Alzheimer's disease, multiple sclerosis, and cancer. As such, finding and exploiting calpain inhibitors is an important area of research.
A team from the Perelman School of Medicine, University of Pennsylvania, in collaboration with the University of California at San Francisco and the Department of Biochemistry and Protein Function Discovery at Queen's University, has developed a unique approach to calpain inhibition by mimicking a natural reaction with a synthesized molecule. The work was published in the latest issue of the Journal of the American Chemical Society.
One of calpain's less beneficial functions is that it eases the ability for cellular invaders such as the Plasmodium falciparum parasite, which is responsible for malaria, to exit their hosts and infect other cells. It is this property that caught the attention of Doron Greenbaum, PhD, assistant professor in of Pharmacology, whose laboratory studies how malaria spreads.
"We have an interest in this protein because it's important for Plasmodium development," he explains. "We initially found that calpain played a role in parasites being able to get out of their host cell, so we became interested in inhibitor development for human calpains."
Greenbaum and his collaborators examined the crystal structure of calpastatin, a naturalcalpain inhibitor, for clues. "We decided to take a different tack on inhibitor development, which has traditionally been designing small peptide-like inhibitors that fit across an enzyme's active site," Greenbaum says. Studying the configuration of how calpastatin bound to the active site of the calpain complex, "we found that there was a small alpha-helix that fit into the active site of the calpain enzyme."
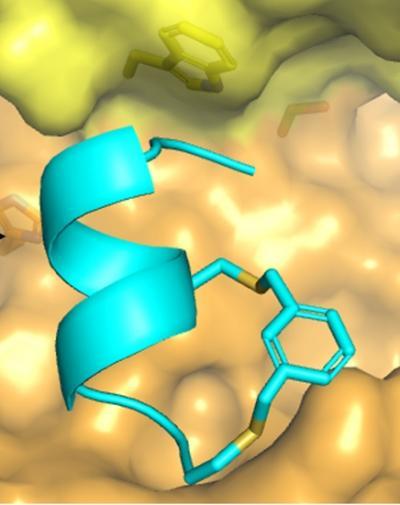
This is a model of a stabilized, alpha-helical inhibitor of the human protease calpain. The blue inhibitor is shown as model of how it is predicted to fit into the protease active site (yellow).
(Photo Credit: Nataline Meinhardt and Doron C. Greenbaum, Perelman School of Medicine, University of Pennsylvania)
Researchers have never before used an alpha-helix structure to inhibit a protease. "Traditionally people thought that alpha-helices normally make horrible inhibitors because it was thought that proteases don't like to bind to them preferring to bind motifs called a beta-sheet," Greenbaum notes. The research team created a peptide with an alpha-helical shape that would fit into the active site of the calpain protease.
The team set out to find a way to stabilize the helix by modifying it with a cross-linking peptide. They screened twenty-four commercially available crosslinkers, identifying five that succeeded in stabilizing the helix. They selected one in particular -- dibromo-m-xylene c15 -- and used it to mimic a protein-protein interaction between calpain and calpastatin. By binding to the active site and thus blocking it, the synthesized molecule inhibits the calpain enzyme from binding with other molecules that permit it to wreak its damaging effects.
"It's the first example of an alpha-helical inhibitor of any protease," Greenbaum says. "Previously no one's ever tried using an alpha-helical motif. It opens up a new way of inhibiting proteases." Aside from being a good inhibitor, the stabilized alpha-helical molecule is also highly specific for calpains, while ignoring other, similar-shaped proteases, thus hopefully downplaying potential side effects if used in humans.
Greenbaum and his collaborators are building upon this initial success to expand the basic concept to a wide range of protease molecules. "The next step is to show how this concept can be generalized to multiple classes of proteases, many of which are pharmaceutically of great interest," he explains. "It's not a single-hit wonder."
The extension of the technique to stabilize the alpha-helix shape in enzymes to other proteins could eventually lead to practical drug therapies for a wide range of diseases, predict the researchers.