Two solids made of the same elements but with different geometric arrangements of the atoms, or crystal phases, can produce materials with different properties. Coal and diamond offer a spectacular example of this effect.
While it is well known that one crystal phase can transform into another under the right circumstances, the mechanisms that facilitate solid-to-solid transitions are still not well understood. Atoms can rearrange themselves to transform from a "parent" phase into a "daughter" phase by two major routes, but it is difficult to predict which route a material will take or why it took one route versus the other.
To this end, researchers from the Hong Kong University of Science and Technology, the University of Pennsylvania, Soochow University in Suzhou, China, and Solvay, have studied colloidal solid-solid transitions with single-particle resolution, and they have discovered a surprising mechanism that facilitates one of these routes. They found that some crystals have an easier time of making the solid-solid transition if they take it in two steps.
Surprisingly, the first step of the process involves the parent phase producing droplets of liquid. The liquid droplets then evolve into the daughter phase.
The observations provide new insight for all sorts of solid-solid phase transformations, and have potential implications for development and manufacture of alloys, as well as natural processes that occur deep within Earth's mantle.
The research team was led by graduate student Yi Peng and associate professor of physics Yilong Han, both of the Hong Kong University of Science and Technology, as well as Arjun Yodh, director of the Laboratory for Research on the Structure of Matter and professor in the Department of Physics and Astronomy in Penn's School of Arts & Sciences.
It was published in Nature Materials.
The two main routes by which a solid-solid phase transition can occur differ by whether the atoms move together or independently of one another. A diffusionless, or martensitic, transition involves many atoms moving cooperatively in unison. This route is often described as a "military" transition, as the atoms "march" in a concerted way. By contrast, a "civilian" transition involves diffusion. It is associated with the formation of "droplets" of the daughter phase within the parent phase, with individual atoms diffusing back and forth in a random manner across the interface between the two phases.
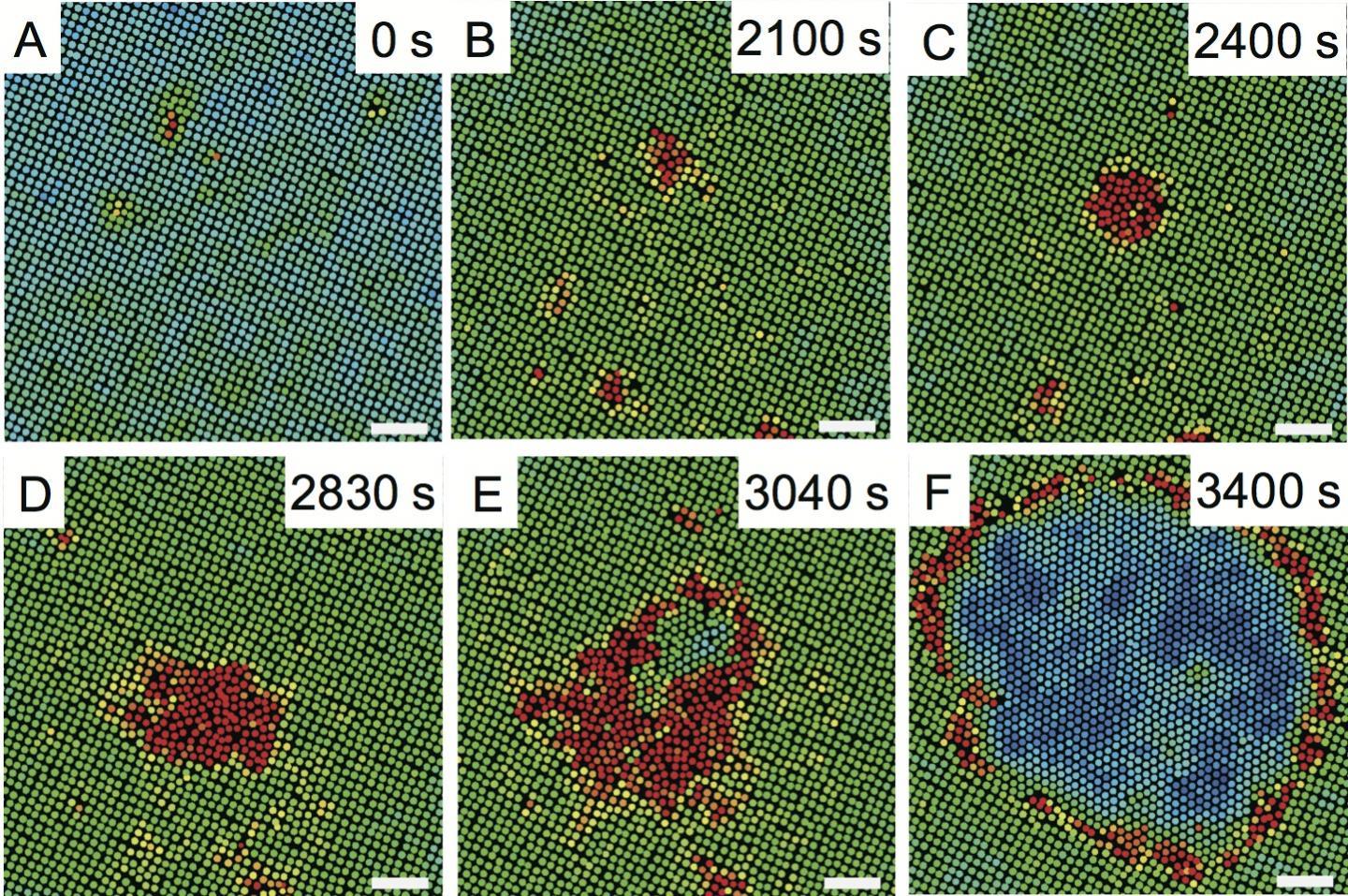
Researchers found that some crystals have an easier time of making solid-solid transitions if they take it in two steps.
Surprisingly, the first step of the process involves the parent phase producing droplets of liquid. The liquid droplets then evolve into the daughter phase. In this series of color-coded images, the square-pattern crystal (labeled green) gives rise to liquid 'droplets' (red), which form triangular crystal (blue).
(Photo Credit: Nature Materials)
"The process we observed is called nucleation," Yodh said. "Particles from the parent phase break away and form droplets of a new phase, and when the droplets get large enough the new crystal phase grows rapidly. What was surprising was that the initial droplets we saw were liquid rather than crystallites."
"A system may not directly transform to the ideal final state if the energy barrier to transformation is high," Han said. "Instead an indirect pathway through some intermediate metastable state with lower barrier height could be more favorable. Such effects can in principle arise in any barrier-crossing process including protein folding, chemical reactions or even some evolutional or social transformations."
The research team devised a way to watch this process in action, using polymer particles synthesized in the Yodh lab that have a unique property: they shrink when heated. The team formed thin films of these particles of a few layers trapped between two transparent walls.
Importantly, the crystalline packing of these spherical particles is highly dependent on the volume occupied in the film by the particles, as well as the ratio of the film thickness to the particle diameter. The solid regions formed by the packing of these spheres had either square or triangular symmetry. The colloidal thin films thus mimicked crystal phases of atoms, and the sample design permitted experimenters to record particle behaviors by video microscopy as they switched from one phase to another.
Because the team was able to shrink the spheres without removing them from the film, simply by shining a heating light on them, they could study the solid-solid phase transition that occurs when the particle size and packing fraction change.
"In our case, the spheres start off in a square lattice," Yodh said, "and, when we shrink them, they transition into a triangular lattice. Such transitions between lattices with different types of lattice symmetries are often difficult to predict, and a liquid intermediate stage has never been suggested in theory before."
With a window into the particles' movements, the team closely observed the process by which this transition occurred. Whether the heated regions of square lattice pattern had defects or not, they found that the transition always exhibited the same basic mechanism. The "colloidal atoms" first formed liquid droplets within the square parent phase, and then a solid triangular crystal phase formed within these liquid droplets. Eventually the triangular crystal phases grew large, replacing both the liquid in the droplets and the parent square phase.
This two-step process, square-crystal to liquid, then liquid to triangular-crystal, was surprising and is potentially indicative of the way in which many solid-solid transitions might occur on the atomic level. The key is that the interfacial energy between the parent crystal and the liquid phase is less than the interfacial energy between the parent crystal and daughter crystal.
"The system first nucleates a liquid, because it costs less interfacial energy than to nucleate the daughter crystal," Yodh said. "The two-step process effectively reduces the energy barrier for the process as a whole."
Source: University of Pennsylvania