A new study by basic science researchers in the Department of Basic Science and Craniofacial Biology at New York University College of Dentistry (NYUCD) sought to understand how gene expression is initiated in the notochord, the evolutionary and developmental precursor of the backbone. The notochord is an axial structure that provides support and patterning signals essential for development in all chordate embryos, including humans.
"A main challenge of modern biology is to understand how specific constellations of genes are switched on in different cells to give rise to distinct tissues," says lead author Dr. Anna Di Gregorio, associate professor in the Department of Basic Science and Craniofacial Biology at NYUCD.
The study, "Brachyury, Foxa2 and the cis-Regulatory Origins of the Notochord," published December 18, 2015, in PLOS Genetics, analyzes the regions of DNA that switch on gene expression in the notochord, called notochord cis-regulatory modules (CRMs, also known as enhancers). The paper presents a systematic analysis of CRMs that share the distinctive property of turning on gene expression in the notochord.
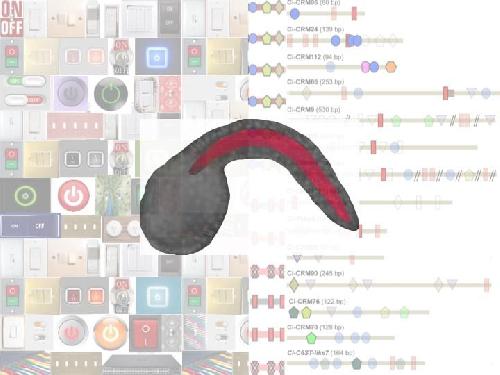 Commonly used electrical switches (background, left) are compared to the molecular switches (cis-regulatory modules, or CRMs) identified in this study (background, right). The horizontal bars represent the 14 CRMs identified in this study, and the colored geometric shapes depict binding sites for transcription factors that are required for these molecular switches to function. The image in the center shows a Ciona embryo, with the notochord highlighted in red. Credit: Dr. Anna Di Gregorio, associate professor in the Department of Basic Science and Craniofacial Biology at NYUCD.
Commonly used electrical switches (background, left) are compared to the molecular switches (cis-regulatory modules, or CRMs) identified in this study (background, right). The horizontal bars represent the 14 CRMs identified in this study, and the colored geometric shapes depict binding sites for transcription factors that are required for these molecular switches to function. The image in the center shows a Ciona embryo, with the notochord highlighted in red. Credit: Dr. Anna Di Gregorio, associate professor in the Department of Basic Science and Craniofacial Biology at NYUCD.
Dr. Di Gregorio and her team used as a model system a marine organism called Ciona (commonly known as "sea squirt"), because it possesses a tractable notochord and a simplified genome, where CRMs can be found faster and more easily. Dr. Di Gregorio notes that systematic studies of CRMs are difficult and time-consuming in humans (and most other chordates), and notochord CRMs have yet to be characterized in humans. Over the past decade using Ciona, the lab has amassed a collection of 34 fully characterized notochord CRMs, the largest in any chordate animal.
In this latest study, the team characterized 14 Ciona notochord CRMs (Fig. 1), isolated the minimal sequences necessary for their function, and then tested whether these minimal sequences could be used to predict related notochord CRMs within the whole Ciona genome. Additionally, they evaluated the evolutionary conservation of CRM sequences between two Ciona species, and compared the structure of the Ciona notochord CRMs to the few fully characterized notochord CRMs identified in other chordates, such as mice and zebrafish.
"While we were analyzing the CRMs of Ciona, we discovered that they are similar to notochord CRMs that had been previously identified in vertebrates," said Dr. Di Gregorio. "This finding is significant because it indicates that this research is not limited to Ciona but extends to other chordates, and most likely humans."
During human development, the notochord is first necessary for the formation of the nervous system and various structures. Later during development, the notochord becomes part of the spine, where it forms a necessary cushion between the vertebrae. The cushy notochord remnants, and the rings that support them, are the structures that can slip (herniate) and cause back pain. While it is known that numerous genes are expressed in the notochord, it is still unclear what turns on gene expression in this particular structure, and how.
The study elucidated that the notochord CRMs contain various DNA sequences that are bound by a particular class of regulatory proteins, called transcription factors. Specific transcription factors bind the CRMs and turn on gene expression in the notochord.
"Despite the differences in the composition of notochord CRMs seen between species--and within the same species--binding sites for two transcription factors, Brachyury and Foxa2, emerged as recurrent hallmarks of notochord CRMs from sea squirts to mice, notes Dr. Di Gregorio. "The present study reports that these transcription factors can work synergistically with each other, but can also ignite notochord gene expression while working either alone or in combination with additional transcription factors of various other families, such as AP1, Sp1/Klf, bHLH, and others."
The researchers note that what little is currently known about human notochord switches stems from indirect evidence, such as computational or biochemical predictions.
"The identification of evolutionarily conserved structural components of these switches for notochord gene expression, such as the binding sites for the regulatory proteins Brachyury and Foxa2," notes Dr. Di Gregorio, "paves the way for the identification of regulatory mutations in other animals with a notochord, including humans. These mutations affect notochord development, and hence the proper formation of the spine."
In further research, Dr. Di Gregorio and her team plan to translate these results to the human genome, where studies of mutations in CRMs, and particularly in notochord CRMs, are still in their early beginnings.
"The ability to identify mutations in notochord CRMs could enable us to predict the occurrence of birth defects, and eventually CRMs could be used as therapeutic targets to correct them," she said.
"There are a growing number of human pathologies that are being traced not to defects in genes, but rather to defects in the molecular switches that control gene expression. Such defects are called 'enhanceropathies,' and our studies contribute to the new field of biomedical research that investigates them," concludes Dr. Di Gregorio.
source: New York University