CRISPR-Cas9's popularity continues to grow ever since its first use in genome editing in January, 2013. What makes CRISPR-Cas9 so remarkable is its astonishing efficiency and availability; relatively speaking, it is easy to use. Last year, scientists at the Center for Genome Engineering within the Institute for Basic Science (IBS) published a paper in Cell Stem Cell describing how they corrected an inverted gene sequence which effectively cured a type of Hemophilia. The Jin-Soo Kim group at IBS has also used CRISPR-Cas9 to perform a process without using foreign DNA to modify crops. It is such an incredibly useful tool and holds so much potential for the future that it was the focus of the International Summit on Human Gene Editing in Washington DC in December 2015. The future of CRISPR applications is only limited by the imaginations of the scientists using it.
CRISPR-Cas9 is an exceptional tool, but the technology isn't perfect. When used, it sometimes produces insertions and deletions, known as indels, at unintended off-target sites. Since unintended gene cleaving could result in an unexpected mutation, it is crucial to detect any errors in the CRISPR-Cas9 process. On January 19th in Genome Research, the Kim team at IBS presented a tool they've dubbed multiplex Digenome-seq (digested genome sequencing), which can map out genome-wide specificities of several CRISPR-Cas9 nucleases simultaneously to find both intentional and unwanted indels quickly and cheaply.
First author Daesik Kim explains that "Digenome-seq differs from other cell-based methods in that it detects DNA cleavages in vitro, rather than in cells, using cell-free genomic DNA." An advantage of using cell-free DNA is that it produces precise analysis results because chromatin, which is normally found in the cell, doesn't interfere with the process. Also, because the DNA in question doesn't need to be amplified en masse using PCR before investigation; Digenome-seq is easier, faster and cheaper to use than previous methods. Digenome-seq works by combining cell-free target DNA with Cas9 protein and sgRNA (single guide RNA) in vitro. The sgRNA leads the Cas9 to the target and off-target site on the DNA where it gets cleaved by the Cas9 endonuclease. In the study, researchers identified on- and off-target sites cleaved in vitro and measured indel frequencies at hundreds of off-target sites in cells which could potentially become unintended mutations. Their accuracy is then scored using a system the IBS team developed called the OTI (off-target effect index) which compares the ratio of off-target to on-target frequencies.
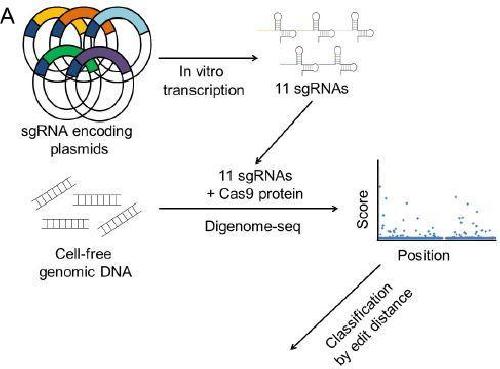 This is a schematic overview of multiplex Digenome-seq. Credit: IBS
This is a schematic overview of multiplex Digenome-seq. Credit: IBS
Unlike previously used monoplex processes that can only work with one sgRNA at a time, multiplex Digenome-seq performs simultaneous analysis of numerous sgRNAs to detect all of their on-target and off-target sites simultaneously. This multiplex process is faster, more efficient and more cost effective than previous methods. In addition to its speed, Digenome-seq is able to detect more off-target indels than other methods like HTGTS or Guide-seq. IBS Director Jin-Soo Kim said "CRISPR-Cas9 is an incredible scientific breakthrough and will continue to be the research focus of biologists around the world." He added that "This technique can be used to choose target sites with the least off-target effects, a method essential for therapeutic applications of CRISPR-Cas9." With superior accuracy, cost savings and speed, multiplex Digenome-Seq appears to be the new standard for testing CRISPR-Cas9 specificity.
source: Institute for Basic Science