WASHINGTON D.C., February 29, 2016 - If you suffer from atrial fibrillation (AF) -- a condition where disorganized electrical signals cause the heart's upper chambers to contract quickly and irregularly -- your doctor may prescribe an antiarrhythmic drug. While these drugs have long been prescribed for AF, which has been linked to an increased risk of stroke, chest pains and even heart failure, their complete mechanisms for restoring action and mitigating these risks have been unclear.
Now, researchers from the Weill Cornell Medical College in New York and the University of Arkansas have new insight into how these drugs work. They tested two types of antiarrhythmic drugs: multi-target drugs, which alter the function of many different cell proteins at once, and single-target drugs, which are designed to affect only one protein.
The researchers found that the multi-target drugs, which are the most commonly prescribed drugs to treat AF and are considered the most efficacious, may work by changing properties of the cell membrane, like elasticity, curvature and thickness.
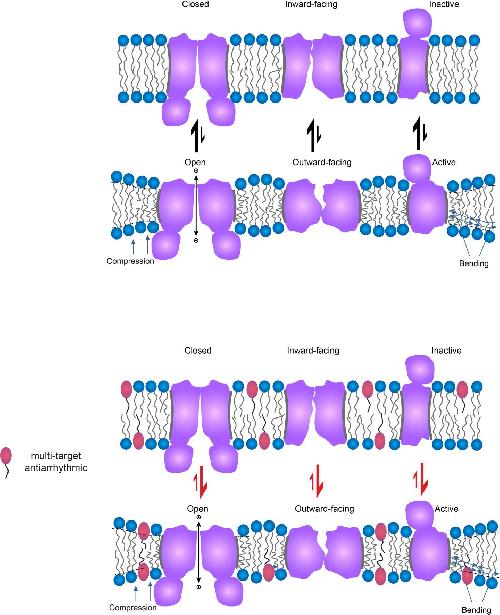 Membrane proteins interact with their host cell membrane, which contributes to their preference for different functional states. This happens because membrane proteins, purple, change shape as they visit different functional states, top vs. bottom panels. When a molecule, such as an antiarrhythmic, the pink ovals with black tails, changes the properties of the cell membrane, represented by the blue circles with two tails, it also changes the membrane's interactions with the embedded proteins and thereby protein function, as indicated by the black arrows vs. red arrows. Therefore, antiarrhythmics that change the properties of bilayer will change function of diverse membrane proteins through this general, membrane-mediated mechanism. Credit: Radda Rusinova/Weill Cornell Medical College
Membrane proteins interact with their host cell membrane, which contributes to their preference for different functional states. This happens because membrane proteins, purple, change shape as they visit different functional states, top vs. bottom panels. When a molecule, such as an antiarrhythmic, the pink ovals with black tails, changes the properties of the cell membrane, represented by the blue circles with two tails, it also changes the membrane's interactions with the embedded proteins and thereby protein function, as indicated by the black arrows vs. red arrows. Therefore, antiarrhythmics that change the properties of bilayer will change function of diverse membrane proteins through this general, membrane-mediated mechanism. Credit: Radda Rusinova/Weill Cornell Medical College
Generating and propagating the electrical impulse that controls heartbeat requires a delicate balance in activities of multiple membrane-embedded proteins. The multiple signaling pathways involved in heartbeat could explain why multi-target drugs could be particularly beneficial in treating conditions such as AF and why the cell membrane may play a key role in their regulation, said Radda Rusinova, a researcher in the Department of Physiology and Biophysics at Weill Cornell Medical College in New York.
Amiodarone, one of the multi-target drugs that Rusinova and her colleagues tested, was initially classified as an antiarrhythmic that prolongs repolarization, which is a "resetting" of the electrical potential across a cell membrane before it can transmit another electrical signal. But other modes of action for the drug were quickly discovered, Rusinova said, and it is now known that amiodarone alters the function of numerous membrane proteins, with no clear mechanism for how it does so.
Rusinova and her colleagues found evidence that a previously unknown membrane-mediated mechanism may be involved in the way the drug changes function of cell membrane-embedded proteins. The researchers used a simplified lipid bilayer with a class of proteins called gramicidin channels embedded in it as a model cell membrane. The gramicidins act as a sort of spy for the researchers. By observing the activity of the gramicidins, the researchers can uncover information about the state of the bilayer.
By using the gramicidin "spies," Rusinova and her colleagues found that amiodarone, along with another multi-target antiarrhythmic drug called dronedarone, increase the elasticity of the bilayer. The elasticity of the bilayer, in turn, affects the function of the proteins embedded within it, similar to the way a changing sea affects all the boats that float on it.
Importantly, the researchers found that this bilayer change occurred within the same range of concentrations of the drug known to be therapeutic. For the single-target antiarrhythmic drugs the team tested, one had almost no effect on the bilayer properties and the other only had an effect at concentrations outside the therapeutic range.
"The key conclusion of this work is that the contribution of bilayer effects on a drug's therapeutic profile is not trivial and has to be carefully examined," Rusinova said.
Rusinova notes the finding could have implications beyond antiarrhythmic drugs. "Our work offers a general mechanism for how drugs alter the function of multiple membrane proteins: drug-induced alterations in lipid bilayer properties result in general changes in membrane protein function."
The poster, "A general mechanism for drug promiscuity: Studies with amiodarone and other antiarrhythmics," by Radda Rusinova, Roger E. Koeppe II and Olaf S. Andersen will be in a poster session starting at 1:45 p.m. PT on Sunday, Feb. 28, 2016 in the West Hall of the Los Angeles Convention Center. ABSTRACT: http://tinyurl.com/hlpw859
source: Biophysical Society