Researchers at the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg and partners in Constance, Munich and Bochum are studying the causes of premature ageing of neurons in Parkinson's patients with a defective DJ1 (PARK7) gene. The genetic defect causes changes in the cellular metabolism meaning that neurons are subjected to oxidative stress and an increased immune response in the brain. The study has just been published in the scientific journal Neurobiology of Disease (DOI: 10.1016/j.nbd.2016.01.019).
Parkinson's disease, the second most common neurodegenerative disease, has genetic causes in 15% of cases. Premature ageing of dopaminergic neurons in the substantia nigra in the brain is the reason for the motor symptoms that characterise this disease. However, how this happens is not yet fully understood. Prof. Karsten Hiller, leader of the "Metabolomics" research group at the LCSB, is looking for the answer in the metabolism. In the current study, he and his research team investigated a specific form of Parkinson's disease with a defective DJ1 gene. "We need the right amount of DJ1. While in some forms of cancer there is too much DJ1, in this case of Parkinson's disease, neurons don't have enough DJ1 and die off", explains Hiller. The research team took a closer look at the metabolism of neurons lacking DJ1 and discovered that two key metabolic pathways were affected. "Without DJ1, neurons cannot absorb enough glutamine and this affects serine production. Both amino acids are important for producing glutathione, which is used to neutralise free radicals", explains Dr. Johannes Meiser, lead author for the study. "In the absence of DJ1, this defence mechanism does not work effectively and oxidative stress occurs. This prematurely ages the cells."
The research team was also able to show that mutations in the DJ1 gene can also negatively affect other cells in the brain. Microglial cells, which are responsible for the immune reaction in the brain, become 'hyperactive' when the DJ1 gene is defective. "Normally, microglial cells are only activated when something in the brain needs to be cleaned up, for instance during inflammation," explains Hiller. "However, if these cells are constantly active, as we discovered happens with the DJ1 defect, this weakens the underlying neurons." Interestingly, the researchers were able to determine metabolic changes not only in the brain's immune cells but also in the blood of Parkinson's patients with mutant DJ1. This could lead to new diagnostic avenues in the future.
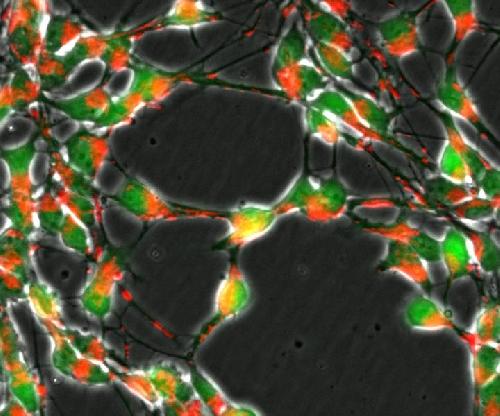 Loss of the DJ1 gene in nerve cells (green) impairs the transport of mitochondria (red) and important metabolic pathways of the cellular respiration. Credit: University of Luxembourg
Loss of the DJ1 gene in nerve cells (green) impairs the transport of mitochondria (red) and important metabolic pathways of the cellular respiration. Credit: University of Luxembourg
The next step will involve investigating how affected metabolic pathways can be influenced using drugs. The changes described in glutamine and serine metabolic processes could thus be used to develop novel approaches for treating Parkinson's.
source: University of Luxembourg