Recent discoveries regarding the physics of ceramic superconductors may help improve scientists' understanding of resistance-free electrical power.
Tiny, isolated patches of superconductivity exist within these substances at higher temperatures than previously were known, according to a paper by Princeton scientists, who have developed new techniques to image superconducting behavior at the nanoscale.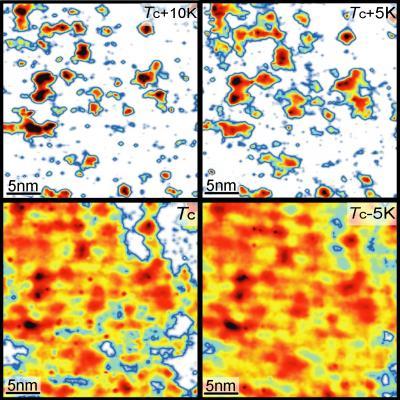 Using a customized microscope, Princeton scientists have mapped the strength of current-carrying electron pairs as they form in a ceramic superconductor. From the top left, the images show the same 30-nanometer square region of the ceramic at successively cooler temperatures. Red areas indicate the presence of superconducting pairs. Even at 10 degrees Celsius above Tc, the temperature at which the entire sample exhibits superconductivity, the electron pairs still exist in localized regions (top left image). Credit: Princeton University/Yazdani labs
Using a customized microscope, Princeton scientists have mapped the strength of current-carrying electron pairs as they form in a ceramic superconductor. From the top left, the images show the same 30-nanometer square region of the ceramic at successively cooler temperatures. Red areas indicate the presence of superconducting pairs. Even at 10 degrees Celsius above Tc, the temperature at which the entire sample exhibits superconductivity, the electron pairs still exist in localized regions (top left image). Credit: Princeton University/Yazdani labs
Superconductivity, the ability to carry electrical current without resistance, could revolutionize electrical power transmission if the property ever appeared in a material at close to room temperature. Even the so-called high-temperature ceramic superconductors discovered two decades ago must be cooled to more than minus 100 degrees Celsius to function.
Using a special customized microscope, the Princeton team has discovered that traces of superconductivity remain present inside these ceramic materials even when they are warmed up above the critical temperature where they lose their resistance. Though the entire sample is too warm to exhibit superconductivity, disconnected regions within it possess Cooper pairs -- the coupled electrons that carry current through a superconductor -- which previously were only known to appear below the critical temperature at which a material superconducts.
The regions are only a few nanometers wide, but they appear in some materials at up to 50 degrees above the critical temperature. Ali Yazdani, senior author of the research paper, said that understanding why these minuscule patches of superconductivity exist at higher temperatures -- and how to create a material that exhibits the property everywhere -- may be the key to enhancing superconductivity.
"Our measurements show that Cooper pairs survive in local patches of the material at temperatures far above the critical temperature," said Yazdani, a professor of physics at Princeton. "Within these tiny regions, there are particular arrangements of atoms that favor formation of electron pairs at very high temperatures. These patches are a precursor to superconductivity and important to enhancing it."
The paper appears in the May 31 edition of Nature. Other members of the research group are Princeton graduate students Kenjiro Gomes and Aakash Pushp and postdoctoral fellow Abhay Pasupathy, as well as Shimpei Ono and Yoichi Ando of the Central Research Institute of Electric Power Industry in Tokyo.
For more than two decades, scientists have worked to explain and enhance the performance of copper-oxide based ceramics, which two decades ago were discovered to superconduct at temperatures far warmer than any other known materials -- though still requiring temperatures that are quite chilly by human standards. High-temperature superconductivity in ceramics has defied a widely accepted explanation and is considered one of the major puzzles in physics.
The key to the puzzle is to determine how electrons, which are negatively charged and normally repel one another, mysteriously change their attitude toward each other and form Cooper pairs. Below the critical temperature, the pairs form everywhere in a material, and can then act in concert as a "superfluid" to carry electric current through it without resistance.
"In lower temperature superconductors, electrons pair up and form a superfluid at the critical temperature -- end of story," Yazdani said. "In ceramics, however, our team is finding that electron pairing occurs over a wide range of temperatures, and their pairing is a function of highly localized chemistry in the sample, often in patches only a few atoms wide."
Investigation on this tiny scale was made possible by a state-of-the-art scanning tunneling microscope the Princeton team designed especially to map superconducting properties on the scale of single atoms while they changed the temperature. The team was able to apply their technique systematically to a large number of high quality copper-oxide superconducting samples.
Unlike an optical microscope that uses light to magnify, the scanning tunneling microscope uses a beam of electrons from a sharp tip to image the sample. The beam served a double purpose for the experiments: Not only does it provide images of a sample down to scales of just a few atoms wide, the beam also is capable of breaking apart electron pairs if it is energetic enough. By varying the energy of the electron beam, the team was able to determine whether pairs had formed in a given spot within the material.
"We spent about two and a half years looking at many different samples at different temperatures to decipher the story," Yazdani said. "We were motivated to search for pairing at high temperatures because of the work of others, most notably that of my colleague Phuan Ong."
The researchers hope to use their experimental results to shed light on what controls the pairing temperature on the atomic scale in ceramic superconductors, and also to determine what limits the Cooper pairs' ability to get their act together to superconduct.
"This type of precision experiment performed while varying temperature gives us a new window into the complex problem of ceramic superconductors," Yazdani said. "If we can figure out the details of what is happening at these local patches within the samples, it might be possible to construct a material that performs better overall."
Such an accomplishment might revolutionize technology for the power industry, said Mike Norman, a physicist in Argonne National Laboratory's Materials Science Division, who was not affiliated with the research.
"If we could raise the critical temperature by making the sample more homogeneous, then superconductivity's application to day-to-day technologies, such as power grids, becomes much more realistic," Norman said. "The nice thing with superconductors is that there is no power loss, so they could be a major player in 'green' and 'efficient' technologies for power transmission."
Source: Visualizing Pair Formation on the Atomic Scale in the High-Tc Superconductor Bi2Sr2CaCu2O8+δBy Kenjiro K. Gomes, Abhay N. Pasupathy, Aakash Pushp, Shimpei Ono, Yoichi Ando and Ali Yazdani