Kyoto University researchers have discovered a way of replacing surface ions of copper oxide nanocrystals at ambient conditions -- a feat that will make nanocage production considerably simpler.
Ionic semiconductor nanocages can be used as photoelectric conversion materials like those used in solar panels. Like a cage in the literal sense, nanocages can also encapsulate drugs and enzymes, promising further developments for targeted drug delivery.
The new method devised by Hsin-Lun Wu and colleagues at Kyoto University exploits preexisting crystal "molds" to make copper oxide nanocrystals morph into hollow copper sulfide nanocages through anion exchange, and ultimately into cadmium sulfide and zinc sulfide nanocages.
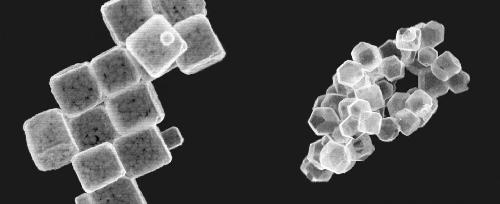 Kyoto University team exploit preexisting crystal "molds" to make copper oxide nanocrystals morph into hollow copper sulfide nanocages through anion exchange, and ultimately into cadmium sulfide and zinc sulfide nanocages. Credit: Kyoto University
Kyoto University team exploit preexisting crystal "molds" to make copper oxide nanocrystals morph into hollow copper sulfide nanocages through anion exchange, and ultimately into cadmium sulfide and zinc sulfide nanocages. Credit: Kyoto University
Nanocages appear in multiple crystal systems depending on their shapes, including cubic and hexagonal systems. Previously, in order to derive hexagonal zinc sulfide nanocages, it was necessary to apply high heat up to around 1000 degrees celcius to zinc sulfide nanocages with a cubic system.
With the Kyoto team's method, all it takes is to expose hexahedral or dodecahedral copper oxide nanocrystals to sodium sulfide; with this process, anions on the surface get replaced, transforming the surface of the nanocrystal to copper sulfide. In addition, the copper oxide in the inside dissolves so as to create a hollow nanocage. When these copper sulfide nanocages are exposed to cadmium nitrate or zinc nitrate, the copper cations become replaced to yield cadmium sulfide nanocages and zinc sulfide nanocages, respectively.
The authors write that such chemical conversions can "overcome the difficulties associated with controlling the size, shape, chemical composition, and crystal structure."
"We never expected that this could be done in such a simple step," says Toshiharu Teranishi, a senior author of the study.
The team hopes to test this method on nanocrystals with various ionic makeup. "Ionic nanocrystals come in so many flavors," said Teranishi. "We're working to find out whether this could be applied as a general method for not just copper oxide nanocrystals, but for other ionic nanocrystals as well."
source: Kyoto University