Researchers from the Massachusetts General Hospital (MGH) Division of Infectious Diseases are investigating the mechanism by which several important pathogenic species of bacteria deliver proteins into the cells of the organisms they are infecting. In a paper receiving advance online publication in Nature Microbiology, the team describes determining a key step in how the diarrheal pathogen Shigella injects proteins into target host cells. Their findings may apply to additional bacterial species, including Salmonella and those responsible for typhoid fever, bubonic plague and many hospital-acquired pneumonias.
"Many bacterial pathogens establish infection by delivering effector proteins - which are responsible for many of the effects of infection - into cells. Therefore, delivery of effector proteins is a significant mediator of the impact of infection on human tissue," says Marcia Goldberg, MD, director of Research in the MGH Division of Infectious Diseases, senior and corresponding author of the report. "The most common mechanism used by a major group of bacteria is a specialized apparatus known as a type 3 secretion system, by which pathogens sitting outside the cell first make a pore in the cell membrane and then deliver effector proteins into the cell. Our data show that interaction between one of the pore proteins and another protein within the cell is required for this process to succeed."
Also called injectisomes, type 3 secretion systems (T3SSs) consist of a base within the pathogen and a needle-like structure that detects contact with the target host cell and through which bacterial proteins associated with the system are secreted. Upon contact with human cells, T3SSs secrete two proteins that make up the pore in the target cell's membrane. After the pore is formed, the T3SS docks onto the pore to deliver bacterial proteins into the cell. Exactly how the pore is formed and the process by which the T3SS docks onto the pore have not been clearly understood, and the MGH team set out to investigate the mechanism behind the docking process.
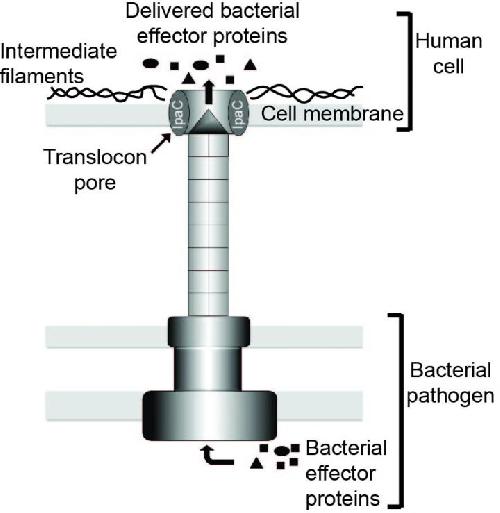 Type 3 secretion systems (T3SSs) are used by several pathogenic bacterial species to deliver effector proteins, responsible for the effects of infection, into the target host cells. An MGH research team has discovered that interaction between structural proteins within the host cell called intermediate filaments and IpaC, a bacterial protein that forms the pore through the host cell membrane, is required for T3SSs to dock onto the pores and secrete effector proteins into the host cell. Credit: (Goldberg Laboratory, Division of Infectious Diseases, Massachusetts General Hospital)
Type 3 secretion systems (T3SSs) are used by several pathogenic bacterial species to deliver effector proteins, responsible for the effects of infection, into the target host cells. An MGH research team has discovered that interaction between structural proteins within the host cell called intermediate filaments and IpaC, a bacterial protein that forms the pore through the host cell membrane, is required for T3SSs to dock onto the pores and secrete effector proteins into the host cell. Credit: (Goldberg Laboratory, Division of Infectious Diseases, Massachusetts General Hospital)
In a series of cellular experiments, they first determined that Shigella infection requires the presence of cytoskeletal proteins called intermediate filaments - in this case a protein called vimentin - within host cells. They then showed that vimentin was required for efficient delivery of effector proteins via the T3SS and that vimentin directly interacts with IpaC, one of the proteins that make up the pores in the host cell membrane. While vimentin is not involved in the formation of pores, the researchers showed it is required for efficient docking of Shigella T3SSs onto the pores in the host cell membrane and that docking induces the secretion of effector proteins into the host cell.
While these experiments focused on the role of vimentin, the investigators also showed that all intermediate filaments normally present in cells infected by Shigella are required for infection by the pathogen. Additional experiments with strains of Salmonella and Yersinia, the families that include the typhoid fever and plague bacteria, confirmed that intermediate filaments were also required for the T3SSs used by those pathogens, suggesting similar mechanisms may apply to all pathogens using T3SSs.
"We know that type 3 secretion systems are critical for the virulence of the bacterial pathogens that use them and their inactivation renders those pathogens non-virulent," says Brian Russo, PhD, a research fellow on Goldberg's team and lead author of the report. "Fundamental understanding of how the type 3 secretion system functions could lead to the discovery of novel therapies for these very important infectious diseases."
source: Massachusetts General Hospital