When a freeway shuts down because of an accident or construction, drivers find another road to take them where they're going. Likewise, when a targeted therapy blocks a pathway that enables tumors to grow, the cells usually manage to get around that obstacle. The result is drug resistance. Researchers have now found a way to map those alternate routes by studying individual cancer cells, suggesting approaches for developing more effective combination therapies. The results are published April 11 in Cancer Cell.
"Because technology now allows us to see the alternate pathways that cancer cells use to drive growth, it will enable us to identify ways to cut off multiple roads at the same time," says James Heath, one of the paper's corresponding authors, at the NanoSystems Biology Cancer Center in the Division of Chemistry and Chemical Engineering at the California Institute of Technology.
In the study, the investigators primarily looked at glioblastoma, the most deadly form of brain cancer. Although therapies tailored based on genetic alterations in these tumors have been developed, their benefit is usually short-lived. Combination therapies, which target multiple alterations at the same time, may offer a better way to fight this disease.
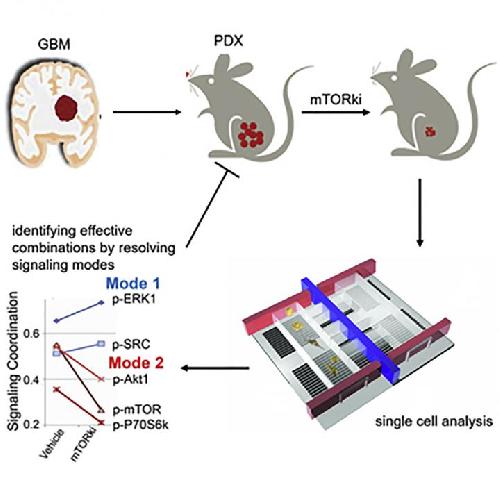 This visual abstract depicts how Wei et al. utilize single-cell phosphoproteomic analysis of patient derived glioblastoma models to identify shifts in signaling coordination following short-term treatment with kinase inhibitors, which facilitates the design of combination therapy approaches with reduced resistance and improved efficacy. Credit: Wei et al./Cancer Cell 2016
This visual abstract depicts how Wei et al. utilize single-cell phosphoproteomic analysis of patient derived glioblastoma models to identify shifts in signaling coordination following short-term treatment with kinase inhibitors, which facilitates the design of combination therapy approaches with reduced resistance and improved efficacy. Credit: Wei et al./Cancer Cell 2016
"Figuring out why resistance to targeted therapies develops has been the focus of our research for a long time," says Paul Mischel, the paper's co-corresponding author, at the Ludwig Institute for Cancer Research at the University of California, San Diego. "In this study, we looked at a drug that should work and found out why it doesn't."
The technology the team used is called single-cell phosphoproteomics. This tool enables investigators to peer into the inner workings of individual cancer cells and see their signaling. Using patient tissues obtained directly from operating rooms, the researchers found that the cells began to adapt to and resist therapies that target the growth pathway called mTOR in as little as 48 hours. Analysis showed that these cells were remapping their routes and finding ways to evade the drug's effect long before any changes could be detected at the clinical level.
The investigators say that this approach could eventually be used to find better combination therapies for glioblastoma, but obstacles remain. "Although the technology used to analyze the cells is relatively simple and inexpensive--just glass and plastic--trials will be difficult to design," says Heath. "For this type of personalized treatment, we won't know what drugs to give patients until after their tumors are analyzed. Every trial will essentially have a sample size of one."
Mischel adds that there are additional challenges in developing drugs for glioblastoma because they must be able to cross the blood-brain barrier.
In the paper, the researchers also described that single-cell phosphoproteomics could be used to study how melanoma cells develop resistance to a class of drugs called BRAF inhibitors. The single-cell-analysis approach could likely be employed to develop personalized treatment for many other types of cancer as well.
source: Cell Press