The offspring of a brooding sea anemone transition from using egg yolks to pre-natal, then post-natal, parental feeding during their development, according to a study published April 22, 2016 in the open-access journal PLOS ONE by Annie Mercier from Memorial University, Canada, and colleagues.
Brooding sea anemones incubate eggs within their central body cavity, later releasing fully formed juveniles, but little is known about the feeding mechanisms used during these stages. The authors of the present study characterized the brooding process of Aulactinia stella, a live-bearing sea anemone found in Atlantic Canada, and compared the fatty acid make-up of adults and juveniles to detect shifts in nutritional resources during development.
They found that parent anemones raised multiple small clutches in their body cavities for a prolonged period, during which the developing clutches underwent remarkable dietary shifts. Initially, all nutrition obtained by the offspring came from yolk contained in the eggs, but juveniles later transitioned to passive nourishment from the parent instead (pre-natal feeding). Once hatched or released juveniles developed functional feeding organs, they began to nurse (post-natal feeding) and ultimately switched to external food sources, no longer dependent on the adult for nutrition.
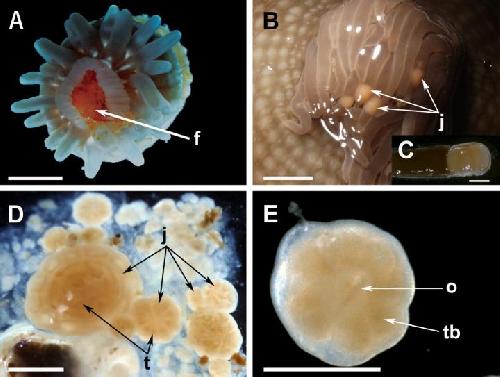 (A) Brooded juvenile; this one was scored as positive for intra-brood feeding based on presence of food (f) in the gastrovascular cavity. (B) Small juveniles (j) moving freely in the tentacles of a brooding adult. (C) Close-up of a small juvenile in B. (D) Size variation of offspring released in a mucus bundle, including tiny propagules and metamorphosing juveniles (j), with primary tentacles (t). (E) Close-up of a small metamorphosing juvenile in D, showing oral pore (o) and tentacle buds (tb). Scale bar represents 2 mm in A, 4 mm in B, 1 mm in C and D, and 0.5 mm in E. Credit: Mercier A et al. PLOS ONE
(A) Brooded juvenile; this one was scored as positive for intra-brood feeding based on presence of food (f) in the gastrovascular cavity. (B) Small juveniles (j) moving freely in the tentacles of a brooding adult. (C) Close-up of a small juvenile in B. (D) Size variation of offspring released in a mucus bundle, including tiny propagules and metamorphosing juveniles (j), with primary tentacles (t). (E) Close-up of a small metamorphosing juvenile in D, showing oral pore (o) and tentacle buds (tb). Scale bar represents 2 mm in A, 4 mm in B, 1 mm in C and D, and 0.5 mm in E. Credit: Mercier A et al. PLOS ONE
In addition, the authors found that the size variation within the clutch may be indications of parent-offspring conflicts or sibling rivalry.
The authors hope that these findings will be useful in exploring the evolution of young-feeding mechanisms, both in cnidarians and in other animals.
source: PLOS