Understanding the complexity of cancer is a major goal of the scientific community, and for kidney cancer researchers this goal just got closer. Dr. Chad Creighton, associate professor of medicine and member of the Dan L Duncan Comprehensive Cancer Center Division of Biostatistics at Baylor College of Medicine, led the study that analyzed close to 900 kidney cancers at the molecular level. The team discovered that what have historically been considered three major types of kidney cancer according to their characteristics under the microscope, could be further distinguished into nine major subtypes through molecular analyses. Each subtype was unique in terms of altered molecular pathways and patient survival. This study made use of data from The Cancer Genome Atlas.
Creighton and colleagues' findings are important because they help pave the way toward more effective personalized medicine. Each kidney cancer has unique characteristics. As a result, different cancers may respond differently to the same treatment. Understanding what makes each kidney cancer unique can provide clues to finding targets for effective therapies. The nine subtypes discovered by Creighton and colleagues were found to have therapeutic implications.
"Different types of cancer can show different pathways being dysregulated," said Creighton. "And for some of the pathways we have therapies we can use to target them."
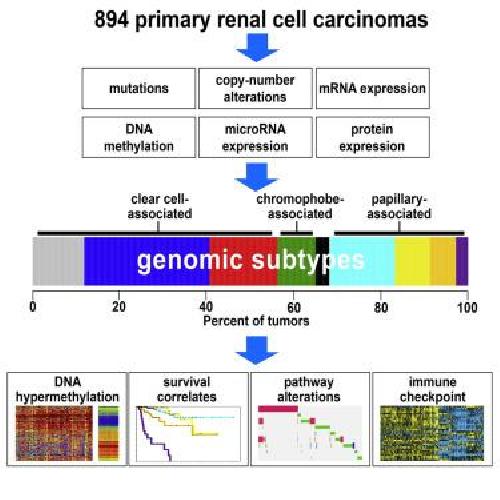 A comprehensive molecular analysis of 894 primary renal cell carcinomas resulted in nine subtypes defined by systematic analysis of five genomic data platforms.Each major histologic types represent substantial molecular diversity. Presumed actionable alterations include PI3K and immune checkpoint pathways. Credit: Dr. Chad Creighton/Cell Reports
A comprehensive molecular analysis of 894 primary renal cell carcinomas resulted in nine subtypes defined by systematic analysis of five genomic data platforms.Each major histologic types represent substantial molecular diversity. Presumed actionable alterations include PI3K and immune checkpoint pathways. Credit: Dr. Chad Creighton/Cell Reports
In particular, the researchers found that a pathway called immune checkpoint was most active in a subtype of clear cell kidney cancer that is typically very aggressive. The immune checkpoint pathway can potentially be a target for specific therapies.
"Not all patients have this pathway activated but molecular analysis would allow us to identify those patients that represent the best candidates for receiving therapies that target that pathway specifically," Creighton said. "If we have this information, then we may have an idea of what would work better for the patient. The molecular information can potentially help guide better decisions for treating each patient."
For Creighton, this work also highlights a rich data resource that he and others expect will be used extensively by investigators in the field to continue advancing their studies of kidney cancer. "Much is going to be learned about kidney cancer with researchers using these data to answer new questions about the disease," Creighton said.
This multi-institutional study was published in Cell Reports.
source: Baylor College of Medicine