The brain is a notoriously difficult organ to treat, but Johns Hopkins researchers report they are one step closer to having a drug-delivery system flexible enough to overcome some key challenges posed by brain cancer and perhaps other maladies affecting that organ.
In a report published online on August 29 in Science Translational Medicine, the Johns Hopkins team says its bioengineers have designed nanoparticles that can safely and predictably infiltrate deep into the brain when tested in rodent and human tissue.
"We are pleased to have found a way to prevent drug-embedded particles from sticking to their surroundings so that they can spread once they are in the brain," says Justin Hanes, Ph.D., Lewis J. Ort Professor of Ophthalmology, with secondary appointments in chemical and biomolecular engineering, biomedical engineering, oncology, neurological surgery and environmental health sciences, and director of the Johns Hopkins Center for Nanomedicine.
After surgery to remove a brain tumor, standard treatment protocols include the application of chemotherapy directly to the surgical site to kill any cells left behind that could not be surgically removed. To date, this method of preventing tumor recurrence is only moderately successful, in part, because it is hard to administer a dose of chemotherapy high enough to sufficiently penetrate the tissue to be effective and low enough to be safe for the patient and healthy tissue.
To overcome this dosage challenge, engineers designed nanoparticles – about one-thousandth the diameter of a human hair – that deliver the drug in small, steady quantities over a period of time. Conventional drug-delivery nanoparticles are made by entrapping drug molecules together with microscopic, string-like molecules in a tight ball, which slowly breaks down when it comes in contact with water. According to Charles Eberhart, M.D., a Johns Hopkins pathologist and contributor to this work, these nanoparticles historically have not worked very well because they stick to cells at the application site and tend to not migrate deeper into the tissue.
Elizabeth Nance, a graduate student in chemical and biomolecular engineering at Hopkins, and Hopkins neurosurgeon Graeme Woodworth, M.D., suspected that drug penetration might be improved if drug-delivery nanoparticles interacted minimally with their surroundings. Nance first coated nano-sized plastic beads of various sizes with a clinically tested molecule called PEG, or poly(ethylene glycol), that had been shown by others to protect nanoparticles from the body's defense mechanisms. The team reasoned that a dense layer of PEG might also make the beads more slippery.
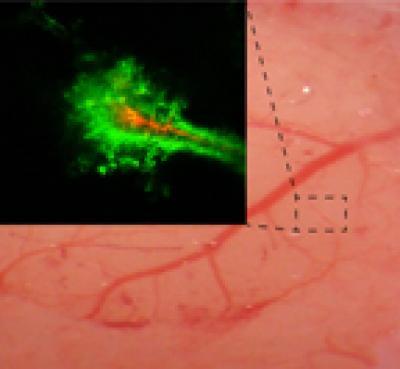
Real-time imaging of a rodent brain shows that nanoparticles coated with polyethylene-glycol (PEG) (green) penetrate farther within the brain than particles without the PEG coating (red).
(Photo Credit: Elizabeth Nance, Graeme Woodworth, Kurt Sailor)
The team then injected the coated beads into slices of rodent and human brain tissue. They first labeled the beads with glowing tags that enabled them to see the beads as they moved through the tissue. Compared to non-PEG-coated beads, or beads with a less dense PEG coating, they found that a dense coating of PEG allowed larger beads to penetrate the tissue, even those beads that were nearly twice the size previously thought to be the maximum possible for penetration within the brain. They then tested these beads in live rodent brains and found the same results.
The researchers then took biodegradable nanoparticles carrying the chemotherapy drug paclitaxel and coated them with PEG. As expected, in rat brain tissue, nanoparticles without the PEG coating moved very little, while PEG-covered nanoparticles distributed themselves quite well.
"It's really exciting that we now have particles that can carry five times more drug, release it for three times as long and penetrate farther into the brain than before," says Nance. "The next step is to see if we can slow tumor growth or recurrence in rodents." Woodworth added that the team "also wants to optimize the particles and pair them with drugs to treat other brain diseases, like multiple sclerosis, stroke, traumatic brain injury, Alzheimer's and Parkinson's." Another goal for the team is to be able to administer their nanoparticles intravenously, which is research they have already begun.
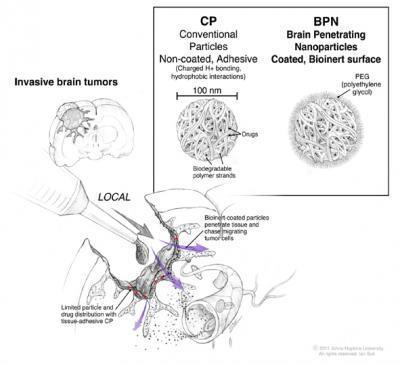
During surgery to excise a brain tumor, direct access to the brain and the space in between its cells is possible. Conventional nanoparticles carrying chemotherapeutic drugs can be applied during the surgery, but they essentially remain on the surface where they are applied (red arrows). Nanoparticles coated with a sufficient amount of PEG have the capacity to diffuse through the tissue (purple arrows) so that they can migrate towards the individual tumor cells that have escaped from the tumor mass.
(Photo Credit: ©2011. Johns Hopkins Department of Neurosurgery. All rights reserved. Ian Suk)
Source: Johns Hopkins Medicine