All tumor cells are the offspring of a single, aberrant cell, but they are not all alike. Only a few retain the capacity of the original cell to create an entire tumor. Such cancer stem cells can migrate to other tissues and become fatal metastases. To fully cure a patient's cancer, it is crucial to find and eliminate all of these cells because any that escape can regenerate the tumor and trigger its spread through the body.
Liang Fang and his colleagues in Walter Birchmeier's group at the Max Delbrück Center for Molecular Medicine (MDC), working with colleagues on the Berlin-Buch campus, have now discovered a molecule that interrupts biochemical signals essential for the survival of a certain type of cancer stem cell. The work is published ahead of print in the online edition of Cancer Research.
In their study Liang and his colleagues focused on a biochemical network within cells called the Wnt signaling pathway, which Birchmeier's lab has studied for many years. One of their discoveries has been that certain types of cancer stem cells require continual stimulation via this pathway to survive and maintain the properties that make them so dangerous. A component of the network called beta-catenin plays an essential role in transmitting Wnt signals to genes that promote the survival and reproduction of the cancer cells. In healthy cells there is no signal from Wnt, and beta-catenin is destroyed.
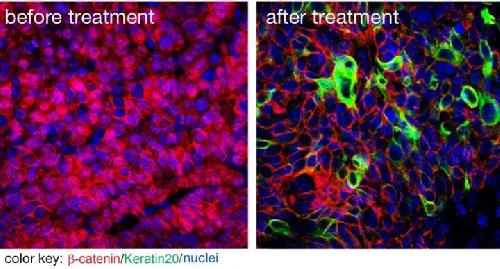 A new inhibitor suppresses tumor growth and cancer stem cells. The image on the left shows beta catenin (red) in cell nuclei indicating that these are cancer stem cells. The image on the right shows that the new substance successfully removed beta catenin from the nuclei. Credit: Picture by Liang Fang for the MDC
A new inhibitor suppresses tumor growth and cancer stem cells. The image on the left shows beta catenin (red) in cell nuclei indicating that these are cancer stem cells. The image on the right shows that the new substance successfully removed beta catenin from the nuclei. Credit: Picture by Liang Fang for the MDC
"In the absence of a signal, beta-catenin is locked out of the cell nucleus," Birchmeier says. "It is linked to a complex of proteins that ultimately break it down. Normally it requires a signal to be released from this 'destruction complex,' and it travels to the cell nucleus." There beta-catenin binds to transcription factors such as the protein TCF4, and in combination the molecules activate specific target genes. In cancer there is no signal, but defective cellular molecules behave as if they have received one and release beta-catenin from the complex.
It might be possible, the scientists reasoned, to prevent this by interrupting the interaction between beta-catenin and TCF4 with a drug. Contacts between two proteins are normally very difficult to destabilize with the small molecules that make up drugs. Proteins usually bind over large areas of their surfaces, which means that a comparatively small obstacle won't prevent the interaction. That is the case with other beta-catenin binding partners.
But the crucial points of contact between beta-catenin and TCF4 appeared to be small "hotspots" which suggested that an inhibitor might block it. Liang Fang took the problem to the campus Screening Unit and Medicinal Chemistry group, a partnership between the MDC and FMP. The facility has high-throughput technology platforms and a "library" of tens of thousands of substances that scientists use to search for inhibitors. The screen turned up a compound they called LF3 which very strongly inhibited binding.
After showing that the compound stripped cancer stem cells of some properties essential to their survival, the lab's next step was to determine whether it would have any effect on tumors in living animals. The scientists turned to the company EPO, a campus-based spin-off of the MDC, to develop lines of mice with tumors derived from human colon cancer tissue. The company specializes in creating mouse models from individual patients' tumors, then testing the animals with a battery of known drugs in hopes of finding one that will effectively combat a specific case of cancer. In this case, all the animals developed tumors, even when injected with a relatively small number of enriched cancer cells.
The animals were then treated with LF3. "We observed a strong reduction of tumor growth," Walter Birchmeier says. "What remained seemed to be completely devoid of cancer stem cells - LF3 seemed to be powerfully triggering these cells to differentiate into benign tissue. At the same time no signaling systems other than Wnt were disturbed. All of these factors make LF3 very promising to further develop as a lead compound, aiming for therapies that target tumors whose growth and survival depend on Wnt signaling."
source: Max Delbrück Center for Molecular Medicine in the Helmholtz Association