One of the world's deadliest mosquitoes sustains its taste for human blood thanks in part to a genetic tweak that makes it more sensitive to human odor, according to new research.
Researchers report in the journal Nature that the yellow fever mosquito contains a version of an odor-detecting gene in its antennae that is highly attuned to sulcatone, a compound prevalent in human odor. The researchers found that the gene, AaegOr4, is more abundant and more sensitive in the human-preferring "domestic" form of the yellow fever mosquito than in its ancestral "forest" form that prefers the blood of non-human animals.
The research provides a rare glimpse at the genetic changes that cause behaviors to evolve, explained first author Carolyn "Lindy" McBride, an assistant professor in Princeton University's Department of Ecology and Evolutionary Biology and the Princeton Neuroscience Institute who conducted the work as a postdoctoral researcher at the Rockefeller University. Uncovering the genetic basis of changes in behavior can help us understand the neural pathways that carry out that behavior, McBride said.
The research also could help in developing better ways to stem the yellow fever mosquito's appetite for humans, McBride said. The yellow fever mosquito is found in tropical and subtropical areas worldwide and is the principal carrier of yellow fever, the measles-like dengue fever, and the painful infection known as chikungunya. Yellow fever annually kills tens of thousands of people worldwide, primarily in Africa, while dengue fever infects hundreds of millions. The research also suggests a possible genetic root for human preference in other mosquitoes, such as malaria mosquitoes, although that species is genetically very different from the yellow fever mosquito.
"The more we know about the genes and compounds that help mosquitoes target us, the better chance we have of manipulating their response to our odor" McBride said, adding that scent is not the only driver of mosquito behavior, but it is a predominant factor.

Researchers report that the yellow fever mosquito sustains its taste for human blood thanks in part to a genetic tweak that makes it more sensitive to human odor. The human-preferring 'domestic' form of the mosquito (right) contains a version of the odor-detecting gene AaegOr4 in its antennae that is highly attuned to sulcatone, a compound prevalent in human odor. The researchers found that this gene is more abundant and more sensitive in the domestic form than in its ancestral 'forest' form (left), which prefers the blood of non-human animals.
(Photo Credit: Carolyn McBride, Department of Ecology and Evolutionary Biology and the Princeton Neuroscience Institute)
The researchers first conducted a three-part series of experiments to establish the domestic yellow fever mosquito's preference for human scent. Forest and domestic mosquitoes were put into a large cage and allowed to bite either a guinea pig or a researcher's arm. Then the mosquitoes were allowed to choose between streams of air that had passed over a guinea pig or human arm. Finally, to rule out general mosquito attractants such as exhaled carbon dioxide, mosquitoes were allowed to choose between the scent of nylon sleeves that had been in contact with a human or a guinea pig.
In all three cases, the domestic form of the yellow fever mosquito showed a strong preference for human scent, while the forest form primarily chose the guinea pig. Although domestic mosquitoes would sometimes go for the guinea pig, it happened very rarely, McBride said.
McBride and colleagues then decided to look for differences in the mosquitoes' antennae, which are equivalent to a human's nose. They interbred domestic and forest mosquitoes, then interbred their offspring to create a second hybrid generation. The genomes of these second-generation hybrids were so completely reshuffled that when the researchers compared the antennae of the human- and guinea pig-preferring individuals they expected to see only genetic differences linked directly to behavior, McBride said.
The researchers found 14 genes that differed between human- and guinea pig-preferring hybrids -- two of them were the odorant receptors Or4 and Or103. Choosing to follow up on Or4, the researchers implanted the gene into fruit-fly neurons. They found that the neurons exhibited a burst of activity when exposed to sulcatone, but no change when exposed to guinea pig odors. McBride plans to further study Or103 and other genes that could be linked to host preference at Princeton.
This work provides insight into how the domestic form of the yellow fever mosquito evolved from its animal-loving ancestor into a human-biting specialist, McBride said. "At least one of the things that happened is a retuning of the ways odors are detected by the antennae," she said. "We don't yet know whether there are also differences in how odor information is interpreted by the brain."
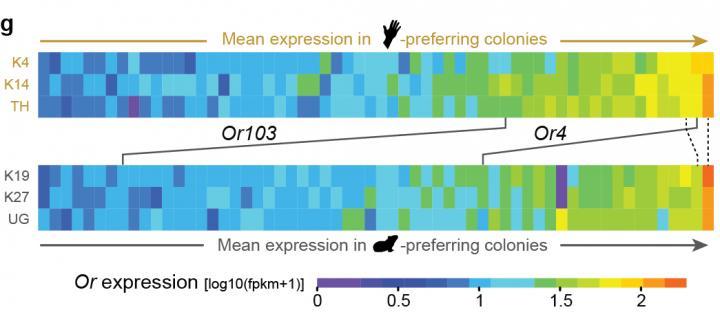
A comparison of domestic and forest form antennae found that two odorant-receptor genes, Or4 and Or103, are more 'expressed,' or abundant, in the human-preferring domestic mosquitoes (top bar) than in the forest form that feeds primarily on non-human animals (bottom bar). The color scale indicates the level of gene expression with purple standing for the least amount and red for the most. The numbers to the left of the colored bars represent three different colonies of each mosquito form. The slanted line under each gene's name points to the level of expression of that gene in each colony.
(Photo Credit: Carolyn McBride, Department of Ecology and Evolutionary Biology and the Princeton Neuroscience Institute)
Source: Princeton University