Fish skin is unique in that it lacks keratin, the fibrous protein found in mammalian skin that provides a barrier against the environment. Instead, the epithelial cells of fish skin are in direct contact with the immediate environment: water. Similarly, the epithelial cells that line the gastrointestinal tract are also in direct contact with their immediate milieu.
"I like to think of fish as an open gut swimming," said J. Oriol Sunyer, a professor in the the Department of Pathobiology of the University of Pennsylvania's School of Veterinary Medicine.
Building on this observation, a study led by Sunyer's group at Penn Vet found that, not only does fish skin resemble the gut morphologically, but key components of skin immune responses are also akin to those of the gut.
"In fish, the skin and the gut have much in common: they are both constantly exposed to environmental insults, they both have a large and varied microbiota and they both contain mucosal surfaces," Sunyer said. "So we hypothesized that the skin should have a similar immune response to the gut, and this is indeed what we found."
The results not only are of interest on the level of basic science and evolution but have important implications for the way that fish vaccines will be designed and tested, as a large number of fish pathogens enter through the skin.
The study was recently featured in the "highlights" section of the September issue of Nature Reviews Immunology, which described it as providing "a fascinating insight into the evolutionary origins of mucosal immune [defenses.]"
The current work is based on a 2010 finding from Sunyer's lab, published in Nature Immunology. In that study, scientists reported for the first time that rainbow trout produce an antibody known as IgT in their gut. This immunoglobulin is responsible for gut mucosal immunity. The equivalent antibody in mammals is IgA.
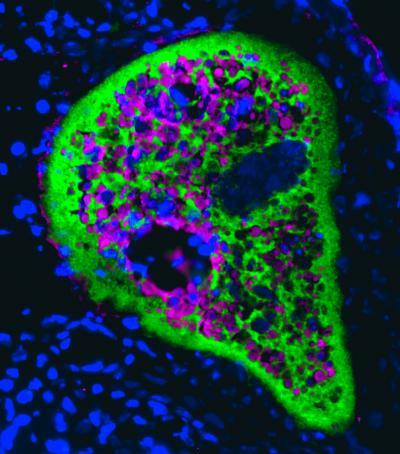
IgT, a fish mucosal immunoglobulin, surrounds a ghost-like Ichthyophtirius multifiliis, a skin parasite of rainbow trout. The parasite is stained in magenta, IgT is stained in green and the cell nuclei are stained with DAPI (blue).
(Photo Credit: Image courtesy of D. G. Atria and J. O. Sunyer, School of Veterinary Medicine, University of Pennsylvania)
Because of the similarities between a fish's gut and skin, Sunyer's team went on the hunt for IgT in the skin tissue of rainbow trout. When they examined B cells, which produce immunoglobulins in response to foreign invaders, such as parasites and bacteria, they found that the majority of B cells in the skin were expressing IgT, suggesting that this immunoglobulin was playing an important role there.
Next the researchers took a closer look at the bacterial community, or microbiota, living on the trout's skin. In mammals and birds, IgA has been found to help prevent the "friendly" bacteria of the gut microbiota from invading the body and causing illness, leading Sunyer's team to hypothesize that IgT might be playing a parallel role in the skin of fish. In addition, earlier work by Sunyer's team found IgT coating bacteria in the intestinal microbiota.
In the current study, when the researchers examined the skin microbiota, they found that a significantly higher percentage of bacteria were coated by IgT than by IgM, another fish immunoglobulin. More critically, greater than 50 percent of the IgT present in the skin mucus was involved in coating bacteria. These findings suggest that IgT is involved in regulating host-microbiota homeostasis; in other words, IgT appears to play a role in maintaining a stable relationship between the fish and the bacterial community living in its skin.
To see how IgT functioned in response to infectious agents, the researchers exposed trout to a parasite that causes white spot disease, a common affliction that targets the skin of farmed, wild and aquarium fish. Compared with uninfected fish, infected fish that survived parasite exposure had many more IgT-producing B cells than IgM-producing B cells in their skin. Moreover, the skin mucus of surviving fish contained only IgT but not IgM, which specifically recognized the parasite. Conversely, IgM represented the main parasite-specific immunoglobulin in the serum of these animals. Taken together, these results demonstrate that IgT is the pivotal skin immunoglobulin generated in response to pathogenic infection.
According to Sunyer, the parallel immune responses in the fish gut and skin are likely the result of these body areas having been subjected to very similar evolutionary selective forces. They also appear to represent an example of convergent evolution with the IgA-mediated mucosal immunity in mammals. In conjunction with earlier work from Sunyer and others, the findings underline that many aspects of mucosal immune responses of fish and mammals operate under the guidance of primordially conserved principles, thus demonstrating the value of bony fish as model organisms.
"Discoveries we make in fish about the fundamental mechanisms of mucosal immunity may help us come up with paradigms of immunity in mammals that are yet to be discovered," Sunyer said. "There is a very important translational component."
The work could also help pave the way for improved fish vaccines — important tools in the burgeoning aquaculture industry.
"The skin is a very important portal for fish pathogens," Sunyer said. "Now that we are starting to understand how mucosal immunity works in the skin and that IgT is the key immunoglobulin there, we can target it and evaluate it when designing new vaccines."
Moving forward, Sunyer's team plans to examine how fish skin's microbiota regulates skin immunity as well as the role of IgT in influencing host-microbiota homeostasis. They also seek to develop new vaccine strategies that will induce IgT immune and protective responses in the skin and other mucosal body parts.
Source: University of Pennsylvania