Our innate immune system, made up mainly of phagocytes, protects our body by exterminating bacteria. To do this, it uses two mechanisms. The first kills foreign bodies within the phagocyte itself. The second kills them outside the cell. These two strategies were already known to researchers, but only in humans and other higher animals. Microbiologists from the University of Geneva (UNIGE), Switzerland, have just discovered that a social amoeba, a unicellular microorganism living in the soils of temperate forests, also uses both these mechanisms, and has done so for over a billion years. Since this amoeba possesses an innate defense system similar to that of humans, while being genetically modifiable, the researchers can therefore carry out experiments on it in order to understand and fight genetic diseases of the immune system. This discovery can be read in the journal Nature Communications.
To defend themselves, our immune cells have two mechanisms. The first, called phagocytosis, kills bacteria within the phagocytic cell itself. The cell envelops the foreign body and exterminates it specifically by using reactive oxygen species (ozone, hydrogen peroxide, bleach), generated thanks to the enzyme NOX2. However, when the invader is too large to be taken up, cells use a second defense mechanism which consists of expelling their genetic material, that is to say their DNA. This DNA transforms into sticky and poisoned nets called «neutrophil extracellular traps» (NETs). These DNA nets then capture bacteria outside of the cell and kill them.
The ancestor of our innate immune system
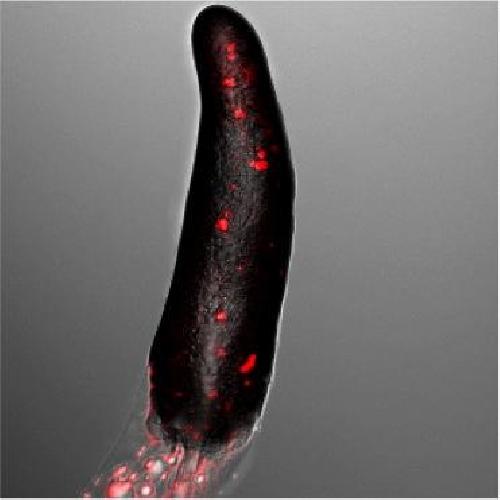 This is a slug made up of social amoebae. In red are the reactive oxygen species produced by the sentinel cells, which are necessary for the generation of DNA nets that defend the slug. Credit: ©Thierry Soldati, UNIGE
This is a slug made up of social amoebae. In red are the reactive oxygen species produced by the sentinel cells, which are necessary for the generation of DNA nets that defend the slug. Credit: ©Thierry Soldati, UNIGE
In collaboration with researchers from Baylor College of Medicine in Huston (USA), Professor Thierry Soldati's team from the Department of Biochemistry of the Faculty of Science at UNIGE studies the social amoeba Dictyostelium discoideum. These microorganisms are bacteria predators. But when food is short, they come together and form a «mini animal» of more than 100,000 cells, called a slug. This will then turn into a «fruiting body» made up of a mass of spores on top of a stalk. Dormant spores will survive without food until the wind or other elements disperse them to new areas where they can germinate and find something to eat.
To make up the slug, approximately 20% of cells sacrifice themselves to create the stalk and 80% will become spores. However, there is a small remaining 1% that keeps its phagocytic functions. «This last percentage is made up of cells called «sentinel» cells. They make up the primitive innate immune system of the slug and play the same role as immune cells in animals. Indeed, they also use phagocytosis and DNA nets to exterminate bacteria that would jeopardize the survival of the slug. We have thus discovered that what we believed to be an invention of higher animals is actually a strategy that was already active in unicellular organisms one billion years ago,» explains Thierry Soldati, last author of the study.
From social amoeba to humans
This discovery plays a primordial role in understanding immune system diseases in humans. Patients with chronic granulomatous disease (CGD) are for example incapable of expressing the functional NOX2 enzyme. Therefore, they suffer recurrent infections, since their immune system lacks the reactive oxygene species that kill bacteria inside the phagosome or via DNA nets. By genetically modifying the social amoeba Dictyostelium discoideum, the microbiologists from UNIGE are able to conduct all sorts of experiments on the mechanisms of the innate immune system. This microorganism can therefore serve as a scientific model for the research on defects in these defense processes, opening the way to possible treatments.
source: Université de Genève