The molecule has proved in preclinical trials to be up to four times more effective than the only pharmacological treatment currently available for familial transthyretin amyloidosis, a rare degenerative disease, and has already been tested in a clinical trial with patients.It acts as a powerful inhibitor of the protein deposits that cause the disease, thus slowing its advance.
Researchers at the Institute of Biotechnology and Biomedicine, Universitat Autònoma de Barcelona (IBB-UAB), in collaboration with the biopharmaceutical company SOM Biotech, located in the Barcelona Science Park (PCB), have published, in Nature Communications, the results of a drug repositioning study in which they describe a powerful drug, SOM0226 (tolcapone) that could significantly improve the pharmacological treatment of familial transthyretin amyloidosis (ATTR).
ATTR is a rare degenerative disease that mainly affects the nervous system and heart muscle tissue (myocardium), and which is usually passed on from parents to children. It originates when the liver and other areas of the organism produce mutations of the protein transthyretin (TTR), which lose their functional structure. This causes toxic aggregates of amyloid fibres to build up, which, depending on the mutation involved, are deposited in different organs, such as the brain, the kidneys, the nerves, the eyes or the myocardium, causing them to malfunction and bringing on the various forms of the disease. To prevent the disease from progressing, a liver transplant or liver and heart transplant is needed.
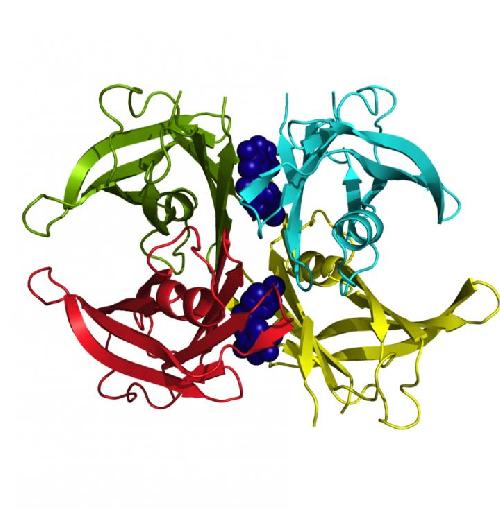 Three-dimensional structure of the TTR mutation that causes familial amyloid cardiomyopathy bound to two tolcapone molecules that stabilise it and prevent it from aggregating. Credit: UAB
Three-dimensional structure of the TTR mutation that causes familial amyloid cardiomyopathy bound to two tolcapone molecules that stabilise it and prevent it from aggregating. Credit: UAB
the study that has just been published, the researchers conducted biophysical trials - in vitro in cell cultures and ex vivo in human plasma and in mouse models of the disease - to show that tolcapone is a powerful inhibitor of the aggregation of amyloid fibres by TTR, stabilising the structure of the protein and thus slowing down the advance of the disease. This is a hitherto unknown property of the drug, which is used to treat Parkinson's disease. The compound turns out to be four times more effective than the only medicine currently available for treating the polyneuropathic variant of ATTR.
The results were positive for all variants of the disease that were studied: familial amyloid polyneuropathy and cardiomyopathy (which affects the peripheral nerves and the myocardium, respectively) and senile systemic amyloidosis, a sporadic form that appears in a very high percentage of men over 60 years of age (and also affects the myocardium). In addition, the treatment was shown to cross the blood-brain barrier, making it the first to tackle the variants that affect the central nervous system.
According to the researchers, this molecule has the potential to become an effective drug for preventing the protein depositions that cause the disease and slowing down its progress, one that could be on the market within five years, as it has already been tested in a clinical trial with persons affected by the neuropathic variant. This trial, led by Dr Josep Gámez, from the Research Institute of the Vall d'Hebron University Hospital, in collaboration with SOM Biotech, was a proof-of-concept test to assess the efficacy and safety of the compound, and it demonstrated the latter's ability to stabilise 100% of TTR in plasma in all the patients treated, to a high degree of safety.
Imitating the thyroid hormone
Tolcapone acts by imitating the process by which the thyroid hormone - T4 or thyroxine - binds to TTR in the bloodstream. Just like the hormone, the drug binds closely to the protein, tying together the four protein sub-units that form the protein's structure. This binding has been proven to stabilise the protein, preventing the sub-units from separating and then forming aggregates.
ATTR is the most common form of familial amyloidosis in the world. In Spain, though the disease can appear anywhere, it is especially prevalent in Palma de Mallorca and Valverde del Camino (Huelva).
Also taking part in the study researchers from the Scripps Research Institute, La Jolla, USA, the Instituto de Biologia Molecular e Celular, Porto, Portugal, and the Instituto Universitario de Investigación de Biocomputación y Física de Sistemas Complejos (BIFI, IQFR-CSIC), Zaragoza.
A repositioned drug
The research was led jointly by Salvador Ventura, a lecturer in the Department of Biochemistry and Molecular Biology of the UAB and a researcher with the IBB, and SOM Biotech, a biopharmaceutical company specialising in drug repositioning, which discovered the use of tolcapone for treating ATTR and holds the patent on it.
Drug repositioning involves taking molecules that have already been approved for a specific therapeutic indication - as is the case with tolcapone for treating Parkinson's disease - and using them for a different disease, thus quickening their development and patients' access to new treatments.
This strategy also helps to lower the cost of treatments, which, in the case of tolcapone, could facilitate its administration in countries like Brazil and Portugal, where the polyneuropathic variant is highly prevalent.
The drug has been designated an orphan drug for ATTR by the American Food and Drug Administration. This is important, as in the United States there is a large group of people suffering from the cardiomyopathic variant of ATTR.
source: Universitat Autonoma de Barcelona