New Monash University research published this week in the Royal Society of Chemistry journal Nanoscale has found a simple and effective way of capturing graphenes and the toxins and contaminants they attract from water by using light. The findings could have significant implications for large-scale water purification.
A small amount of a special light-sensitive soap was added to the water containing the graphenes and contaminants. The soap changes its molecular structure when light of a particular colour is shone onto it. This changes the way it interacts with carbon materials in the graphene and causes them to separate out (along with contaminants stuck to them), enabling easier extraction of the graphenes and contaminants. Shining a different coloured light re-disperses the graphenes for re-use.
Monash researcher Dr Rico Tabor explained the diverse technological opportunities offered by graphene owing to its unique structure and properties.
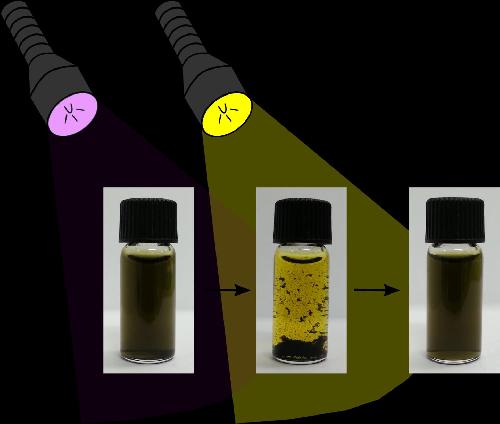 By simply shining the right color of light on the graphene, contaminants and light-sensitive soap mixture, the graphene clusters together and sinks; shining a different color of light re-disperses it for re-use. Credit: Monash University
By simply shining the right color of light on the graphene, contaminants and light-sensitive soap mixture, the graphene clusters together and sinks; shining a different color of light re-disperses it for re-use. Credit: Monash University
"Among its many potential uses, the prospect of using graphenes for the purpose of water purification is extremely promising. Because the structure is essentially two-dimensional and only an atom thick, graphene `sheets' have the highest surface area possible, meaning their capacity to mop up contaminants in water surpass that of any currently used materials or membranes," Dr Tabor said.
"However, this raises the problem of how to extract the graphenes and contaminants from water. Traditional approaches use high amounts of energy by centrifugation, or adding large amounts of polymer at high cost," Dr Tabor said.
Co-researcher Thomas McCoy explained the significance of these latest research findings and the benefits of using light to capture graphenes.
"Light is appealing as it is abundantly available, simple and low cost when compared to most separation methods. Our latest research findings could have significant implications for cost-effective, large-scale water treatment," Mr McCoy said.
source: Monash University