A new technique for creating films of barium titanate (BaTiO3) nanoparticles in a polymer matrix could allow fabrication of improved capacitors able to store twice as much energy as existing devices. The improved capacitors could be used in consumer devices such as cellular telephones – and in defense applications requiring both high energy storage and rapid current discharge.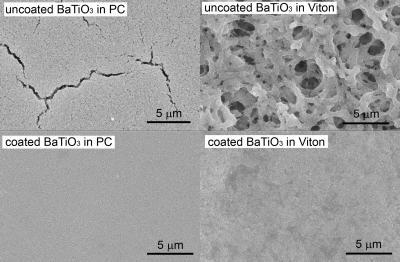 Scanning electron micrographs of barium titanate (BaTiO3) nanocomposites with polycarbonate (left, top and bottom) and Viton (right, top and bottom) polymer matrices. The images show the dramatic improvement in film uniformity through the use of phosphonic acid coated BaTiO3 nanoparticles (bottom images) as compared to uncoated nanoparticles (top images). Credit: Image courtesy of Joe Perry
Scanning electron micrographs of barium titanate (BaTiO3) nanocomposites with polycarbonate (left, top and bottom) and Viton (right, top and bottom) polymer matrices. The images show the dramatic improvement in film uniformity through the use of phosphonic acid coated BaTiO3 nanoparticles (bottom images) as compared to uncoated nanoparticles (top images). Credit: Image courtesy of Joe Perry
Because of its high dielectric properties, barium titanate has long been of interest for use in capacitors, but until recently materials scientists had been unable to produce good dispersion of the material within a polymer matrix. By using tailored organic phosphonic acids to encapsulate and modify the surface of the nanoparticles, researchers at the Georgia Institute of Technology’s Center for Organic Photonics and Electronics were able to overcome the particle dispersion problem to create uniform nanocomposites.
"Our team has developed nanocomposites that have a remarkable combination of high dielectric constant and high dielectric breakdown strength," said Joseph W. Perry, a professor in the Georgia Tech School of Chemistry and Biochemistry and the Center for Organic Photonics and Electronics. "For capacitors and related applications, the amount of energy you can store in a material is related to those two factors."
The new nanocomposite materials have been tested at frequencies of up to one megahertz, and Perry says operation at even higher frequencies may be possible. Though the new materials could have commercial application without further improvement, their most important contribution may be in demonstrating the new encapsulation technique – which could have broad applications in other nanocomposite materials.
"This work opens a door to effectively exploit this type of particle in nanocomposites using the coating technology we have demonstrated," explained Perry. "There are many ways we can envision making advances beyond what we’ve done already."
The results were reported in the April 2007 edition (Vol. 19, issue 7) of the journal Advanced Materials. The research was supported by the Office of Naval Research and the National Science Foundation. Georgia Tech has filed a patent application on the nanoparticle encapsulation technique.
Because of their ability to store and rapidly discharge electrical energy, capacitors are used in a variety of consumer products such as computers and cellular telephones. And because of the increasing demands for electrical energy to power vehicles and new equipment, they also have important military applications.
Key to developing thin-film capacitor materials with higher energy storage capacity is the ability to uniformly disperse nanoparticles in as high a density as possible throughout the polymer matrix. However, nanoparticles such as barium titanate tend to form aggregates that reduce the ability of the nanocomposite to resist electrical breakdown. Other research groups have tried to address the dispersal issue with a variety of surface coatings, but those coatings tended to come off during processing – or to create materials compatibility issues.
The Georgia Tech research team decided to address the issue by using organic phosphonic acids to encapsulate the particles. The tailored organic phosphonic acid ligands, designed and synthesized by a research group headed by Seth Marder – a professor in the Georgia Tech School of Chemistry and Biochemistry – provide a robust coating for the particles, which range in size from 30 to 120 nanometers in diameter.
"Phosphonic acids bind very well to barium titanate and to other related metal oxides," Perry said. "The choice of that material and ligands were very effective in allowing us to take the tailored phosphonic acids, put them onto the barium titanate, and then with the correct solution processing, to incorporate them into polymer systems. This allowed us to provide good compatibility with the polymer hosts – and thus very good dispersion as evidenced by a three- to four-fold decrease in the average aggregate size."
Though large crystals of barium titanate could also provide a high dielectric constant, they generally do not provide adequate resistance to breakdown – and their formation and growth can be complex and require high temperatures. Composites provide the necessary electrical properties, along with the advantages of solution-based processing techniques.
"One of the big benefits of using a polymer nanocomposite approach is that you combine particles of a material that provide desired properties in a matrix that has the benefits of easy processing," Perry explained.
Though the new materials may already offer enough of an advantage to justify commercializing, Perry believes there are additional opportunities for boosting their performance. The research team also wants to scale up production to make larger samples – now produced in two-inch by three-inch films – available to other researchers who may wish to develop additional applications.
Perry and Marder are working with Bernard Kippelen, a professor in the Georgia Tech School of Electrical and Computer Engineering, on the use of these new nanocomposites in organic thin-film transistors in which solution-based techniques are used to fabricate inexpensive electronic components.
"Beyond capacitors, there are many areas where high dielectric materials are important, such as field-effect transistors, displays and other electronic devices," Perry added. "With our material, we can provide a high dielectric layer that can be incorporated into those types of applications."
The research team also included Philseok Kim, Simon Jones, Peter Hotchkiss and Joshua Haddock.