PASADENA, Calif.—Researchers at the California Institute of Technology (Caltech) have obtained the first high-resolution, three-dimensional images of a cell with a nucleus undergoing cell division. The observations, made using a powerful imaging technique in combination with a new method for slicing cell samples, indicate that one of the characteristic steps of mitosis is significantly different in some cells.
During mitosis, two sets of chromosomes get paired up at the center of the cell's nucleus. Then hollow rods of proteins called microtubules, which make up a cellular structure called the spindle apparatus, grab on to the chromosomes and essentially pull each set away from the center in opposite directions, so that both daughter cells end up with a full copy of the genetic material. Typically, in the cells of plants, fungi, and many animals, one or more microtubules attach to each chromosome before the spindle will separate the sets of chromosomes from each another.
But when the Caltech researchers observed this step using their new technique, what they saw was not business as usual for a dividing cell. "We've found the first clear example of a cell where there are fewer microtubules used than chromosomes," says Grant Jensen, professor of biology at Caltech and a Howard Hughes Medical Institute investigator. The group's findings appear online in Current Biology and will be published in the September 27 issue of the journal.
Jensen's group is one of just a few in the world that uses electron cryotomography (ECT) to image biological samples. Unlike traditional electron microscopy—for which samples must be dehydrated, embedded in plastic, sectioned, and stained—ECT involves plunge-freezing samples so quickly that they become trapped in a near-native state within a layer of transparent, glasslike ice. A microscope can then capture high-resolution images of the sample as it is rotated, usually one degree at a time.
One limitation of ECT is that samples cannot be thicker than 500 nanometers—otherwise the electron beam cannot penetrate the sample sufficiently. Therefore, ECT studies have focused on small bacteria and viruses. But Jensen's group wanted to extend the technique to observe eukaryotic cells, which are typically much bigger. So they located the smallest known eukaryote, Ostreococcus tauri, and imaged it with ECT.
The next step was to observe the important process of cell division in a eukaryote. But even tiny O. tauri exceeds the 500-nanometer limit when it is undergoing mitosis, since cells are essentially twice as big as usual when they're dividing. So the researchers needed a way to cut the frozen sample into slices, a process called cryosectioning.
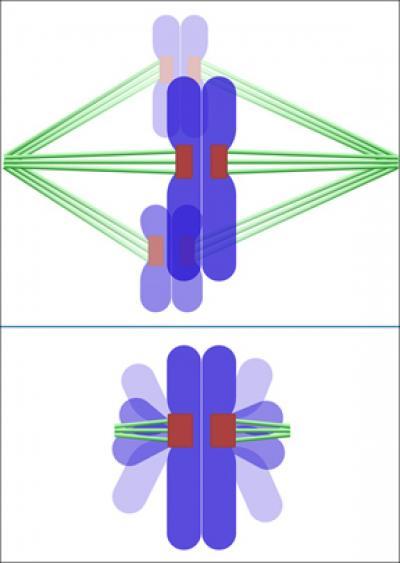
A "regular" spindle configuration (top) with all chromosomes attached to microtubules, and the O. tauri spindle (bottom) with chromosomes bundled together and attached to just two short, incomplete microtubules.
(Photo Credit: Caltech)
"In the past when people have tried this, the sections have come off sort of like snowflakes—the material has gotten crushed," Jensen says. But Caltech electron microscopy scientist Mark Ladinsky has developed a highly successful new technique for cryosectioning samples. He slices them at about -150°C, using a special machine and a diamond knife. Then he carefully removes the slices from the knife using a micromanipulator. The technique has enabled the researchers to look at slices through dividing cells in a near-native, hydrated state.
With the new imaging and sectioning techniques working together, a former postdoctoral scholar in Jensen's lab, Lu Gan, was able to make detailed observations of mitosis in O. tauri, a cell with 20 chromosomes. Contrary to expectations, Gan observed nowhere near 40 microtubules attached to the two sets of chromosomes during mitosis; instead, he found only about 10 small, incomplete microtubules. This suggests that the chromosomes may link together to form some kind of a bundle that can then be segregated all at once by a smaller number of microtubules.
Previous studies, dating back to the 1970s, claimed to have found unicellular eukaryotic cells with fewer microtubules than chromosomes. However, those cells were chemically fixed and stained—a process that can easily damage the cells—casting doubt on the claims. But with their new imaging and sectioning techniques, the Caltech researchers feel confident that they have indeed imaged such a cell.
Their success bodes well for future studies. "Our work with O. tauri shows that we might be able to get high-resolution, three-dimensional images of other eukaryotic cells, which are much larger than bacteria," Jensen says. "We've even moved on to try to image some human cells using the same process."

Caltech professor of biology and Howard Hughes Medical Institute investigator Grant Jensen with his electron cryotomography (ECT) set up. Jensen's group is one of just a few in the world that uses ECT to capture high-resolution images of biological samples in a near-native, hydrated state.
(Photo Credit: Caltech)