Because concrete, the world's most-used construction material, is such a major contributor to climate change, it's worth knowing every detail about how it's manufactured, according to Rice University scientists.
The Rice lab of theoretical physicist Rouzbeh Shahsavari is looking into those details down to the atomic level. The lab has published the results of computer modeling studies that detail how dislocations - screw-like defects - in raw crystals used for concrete influence how efficiently it can be made.
The research published this month in the Journal of the American Ceramic Society shows that tricalcium silicates (C3S) that consist of pure rhombohedral crystals are better than others for producing "clinkers." Clinkers are round lumps of C3S that, when ground into a powder, mix with water to make cement, the glue that holds gravelly concrete together. The easier a clinker is to grind, the less energy a manufacturer needs to grind it.
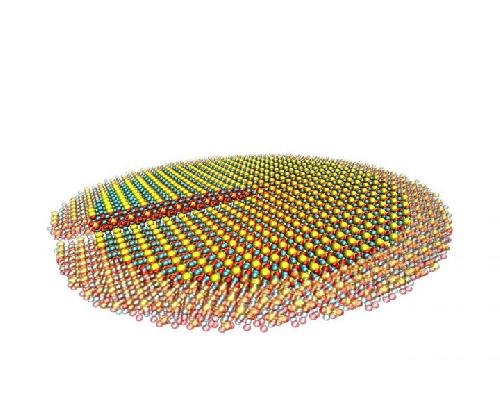 A computer model shows tricalcium silicate with a defect known as a screw dislocation, which influences the brittle properties of the crystal structure. The atoms on the periphery are faded because they are little affected by the dislocation core at the center of the disc. Credit: Lei Tao/Rice University
A computer model shows tricalcium silicate with a defect known as a screw dislocation, which influences the brittle properties of the crystal structure. The atoms on the periphery are faded because they are little affected by the dislocation core at the center of the disc. Credit: Lei Tao/Rice University
Last year, the Shahsavari lab reported that hot clinkers were easier to grind. They also looked at the detrimental effects of screw dislocations on how well the resulting powder mixes with water.
This time, the lab built computer models of the molecular structures that make up several commonly used types of C3S to see which were prone to be more brittle, despite the inevitable dislocations that twist the crystals into unpredictable formations. (The more brittle, the better.)
They also sought to understand how defects in the microscopic crystals influence the powder's ability to react with water and found that rhombohedrals are also more reactive to water than the two monoclinic clinkers they studied. Rhombohedral crystals have edges that are all the same length; monoclinic crystals do not.
"Understanding and quantifying the structure, energetics and the effect of defects on mechanics and reactivity of cement crystals is a fundamental and engineering challenge," Shahsavari said. "This work is the first study that puts an atomistic lens on the key characteristics of screw dislocations, a common line defect in C3S, which is the main ingredient of Portland cement."
Shahsavari insisted that maximizing energy use in the production of concrete is important, an attitude supported by attendees at last year's climate summit in Paris. He noted at the time that the annual worldwide production of more than 20 billion tons of concrete contributes 5 to 10 percent of carbon dioxide to global emissions, surpassed only by transportation and energy generation as a producer of greenhouse gas.
Besides the findings of this work, this study paves the path to investigate such other defects as edge dislocation, brittle-to-ductile transitions and twinning deformations in cement, Shahsavari said. Together, these will provide new physical insights and strategies to tune the grinding energy and reactivity of cement, which would lower the energy consumption and carbon dioxide footprint associated with concrete.
source: Rice University