A stem cell capable of regenerating both bone and cartilage has been identified in bone marrow of mice. The discovery by researchers at Columbia University Medical Center (CUMC) is reported today in the online issue of the journal Cell.
The cells, called osteochondroreticular (OCR) stem cells, were discovered by tracking a protein expressed by the cells. Using this marker, the researchers found that OCR cells self-renew and generate key bone and cartilage cells, including osteoblasts and chondrocytes. Researchers also showed that OCR stem cells, when transplanted to a fracture site, contribute to bone repair.
"We are now trying to figure out whether we can persuade these cells to specifically regenerate after injury. If you make a fracture in the mouse, these cells will come alive again, generate both bone and cartilage in the mouse--and repair the fracture. The question is, could this happen in humans," says Siddhartha Mukherjee, MD, PhD, assistant professor of medicine at CUMC and a senior author of the study.
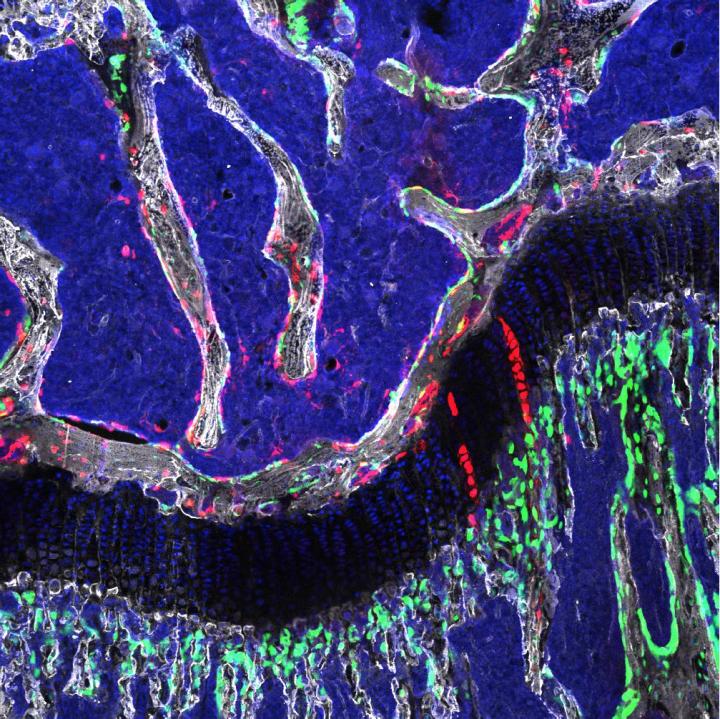
Osteochondroretricular stem cell, a newly identified type of bone stem cell that appears to be vital to skeletal development and may provide the basis for novel treatments for osteoarthritis. Credit: Laboratory of Dr. Timothy Wang
The researchers believe that OCR stem cells will be found in human bone tissue, as mice and humans have similar bone biology. Further study could provide greater understanding of how to prevent and treat osteoporosis, osteoarthritis, or bone fractures.
"Our findings raise the possibility that drugs or other therapies can be developed to stimulate the production of OCR stem cells and improve the body's ability to repair bone injury--a process that declines significantly in old age," says Timothy C. Wang, MD, the Dorothy L. and Daniel H. Silberberg Professor of Medicine at CUMC, who initiated this research. Previously, Dr. Wang found an analogous stem cell in the intestinal tract and observed that it was also abundant in the bone.
"These cells are particularly active during development, but they also increase in number in adulthood after bone injury," says Gerard Karsenty, MD, PhD, the Paul A. Marks Professor of Genetics and Development, chair of the Department of Genetics & Development, and a member of the research team.
The study also showed that the adult OCRs are distinct from mesenchymal stem cells (MSCs), which play a role in bone generation during development and adulthood. Researchers presumed that MSCs were the origin of all bone, cartilage, and fat, but recent studies have shown that these cells do not generate young bone and cartilage. The CUMC study suggests that OCR stem cells actually fill this function and that both OCR stems cells and MSCs contribute to bone maintenance and repair in adults.
The researchers also suspect that OCR cells may play a role in soft tissue cancers.
The paper is titled, "Gremlin 1 identifies a skeletal stem cell with bone, cartilage and reticular stromal potential." The other contributors are Daniel L. Worthley (CUMC, University of Adelaide, SA, Australia, South Australian Health and Medical Research Institute, SA, Australia, and Royal Children's Hospital, Vic., Australia), Michael Churchill (CUMC), Jocelyn T. Compton (CUMC), Yagnesh Tailor (CUMC), Meenakshi Rao (CUMC), Yiling Si (CUMC), Daniel Levin (Keck School of Medicine of the University of Southern California, CA), Matthew G. Schwartz (Harvard Medical School, Cambridge, MA), Aysu Uygur (Harvard), Yoku Hayakawa (CUMC), Stefanie Gross (CUMC), Bernhard W. Renz (CUMC), Wanda Setlik (CUMC), Ashley N. Martinez (CUMC), Xiaowei Chen (CUMC), Saqib Nizami (CUMC), Heon Goo Lee (CUMC), H. Paco Kang (CUMC, Jon-Michael Caldwell (CUMC), Samuel Asfaha (CUMC), C. Benedikt Westphalen (CUMC and University Hospital Munich, Ludwig-Maximilians-University Munich - Campus Groβhadern, Munich, Germany), Trevor Graham (Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK), Guangchun Jin (CUMC), Karan Nagar (CUMC), Hongshan Wang (CUMC), Mazen A. Kheirbek ( CUMC), Alka Kolhe (CUMC), Jared Carpenter (CUMC), Mark Glaire (CUMC), Abhinav Nair (CUMC), Simon Renders (CUMC), Nicholas Manieri (Washington University in St Louis, MO), Sureshkumar Muthupalani (Massachusetts Institute of Technology, Cambridge, MA), James G. Fox (MIT), Maximilian Reichert (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA), Andrew S. Giraud (CUMC), Robert F. Schwabe (CUMC)), Jean-Phillipe Pradere (CUMC and Université Paul Sabatier, Institut des Maladies Métaboliques et Cardiovasculaires, Toulouse, France), Katherine Walton (University of Michigan, Ann Arbor, MI), Ajay Prakash (Michigan), Deborah Gumucio (Michigan), Anil K. Rustgi (Pennsylvania), Thaddeus S. Stappenbeck (Washington), Richard A. Friedman (CUMC)), Michael D. Gershon (CUMC), Peter Sims (CUMC), Tracy Grikscheit (Keck School of Medicine of the University of Southern California, Los Angeles, CA), and Francis Y. Lee (CUMC).
The authors declare no financial or other conflicts of interest.
The study was funded by grants from the National Institutes of Health (5U54 CA126513, R01 RHL115145A, AR056246, and EB006834),), the Robert Carroll and Jane Chace Carroll Laboratories, the American Cancer Society, the NH&MRC and Menzies Foundation, Cancer Council SA's Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health, Gastroenterological Society of Australia, the American Gastroenterological Association, the American Association for Cancer Research, the Royal Australasian College of Physicians, and the Columbia University Ines Mandl Postdoctoral research fellowship.