This news release is available in German.
Scientists at the University of Oulu, Finland, and at the Helmholtz Center Berlin (HZB) have shown the way to new directions in drug development against African sleeping sickness and other tropical parasitic infections. This was based on the structural analysis of the enzyme thiolase, which plays a central role in lipid metabolism in the parasite that causes sleeping sickness. The researchers examined the biomolecule's structure at the MX beamline of electron storage ring, BESSY II, at the HZB. (Biochemical J. 2013, DOI: 10.1042/BJ20130669)
Sleeping sicknesses – african trypanosomiasis, kala-azar, indian leishmaniasis – are infections caused by tropical parasites. Millions get sick from them each year and thousands end up dying. Anti-parasitic drugs are expensive and often have a host of unwanted side effects. In decades, there have been no new effective therapies. Reason enough for the World Health Organization (WHO) to consider research, which can lead to the development of new anti-parasitic drugs, a top priority.
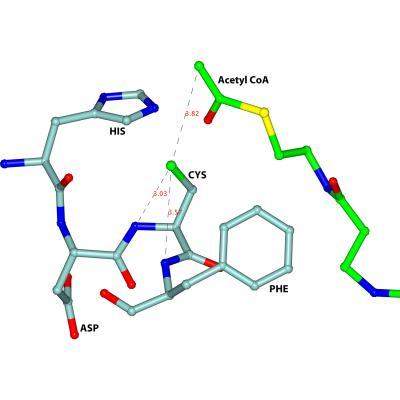
A key feature of the active site of the trypanosomal thiolase is the HDCF-loop (HIS-ASP-CYS-PHE), visualised in light blue.
(Photo Credit: Image: University of Oulu)
Now, Prof. Rik Wierenga and his team at Oulu University have paved the way for this type of research by shedding light on the structure of the enzyme thiolase. Thiolase figures prominently in parasitic lipid metabolism. According to Wierenga, "key is knowing the geometry of the enzyme's active site. This is the place where lipids that play a central role in parasitic metabolism attach and where chemical reactions that convert lipids into other substances take place." Which is why it's important to investigate the active site's structure and function: "It enables us to develop lipid-like substances that firmly attach to the active site and block it." The molecules that are involved represent the ideal starting points for new drug development.
Studies at BESSY of the enzyme thiolase have yielded a highly detailed image of thiolase's active site. "We now have a much clearer idea of thiolase's role in all this," says Wierenga. "It would appear that the enzyme catalyzes the first step in the sterol biosynthesis pathway, which is important in a number of parasites."
"The measurements of crystalline thiolase proteins we obtained at our MX beamline has helped to unravel the active site's geometry," says HZB's own Dr. Manfred Weiss. One particular region of the protein called the HDCF loop turns out to be key. The structure, which lies deep within thiolase's interior, was previously unknown. "Understanding the HDCF loop is the ideal starting point for the development of new anti-parasitic drugs," adds Wierenga.
Source: Helmholtz-Zentrum Berlin für Materialien und Energie