Researchers at Purdue University and The Catholic University of America have discovered the structure of an enzyme essential for the operation of "molecular motors" that package DNA into the head segment of some viruses during their assembly.
The enzyme, called an ATPase, provides energy to run the motor needed to insert DNA into the capsid, or head, of the T4 virus, which is called a bacteriophage because it infects bacteria. The same kind of motor, however, also is likely present in other viruses, including the human herpes virus.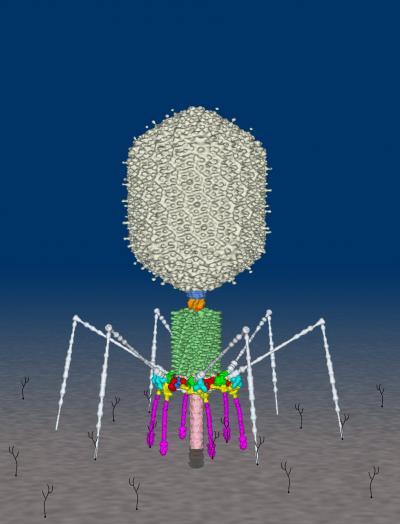 This image depicts the structure of the T4 virus, called a bacteriophage because it infects bacteria. Researchers at Purdue and The Catholic University of America have discovered the structure of an enzyme called an ATPase, which is essential for the operation of "molecular motors" that package DNA into the head segment of the T4 virus. The same kind of motor, however, is also likely present in other viruses, including the human herpes virus.Credit: Frederick A. Eiserling/UCLA and Petr Leiman/Purdue Department of Biological Sciences
This image depicts the structure of the T4 virus, called a bacteriophage because it infects bacteria. Researchers at Purdue and The Catholic University of America have discovered the structure of an enzyme called an ATPase, which is essential for the operation of "molecular motors" that package DNA into the head segment of the T4 virus. The same kind of motor, however, is also likely present in other viruses, including the human herpes virus.Credit: Frederick A. Eiserling/UCLA and Petr Leiman/Purdue Department of Biological Sciences
"This is the first time a structure has been determined of an ATPase involved in a DNA packaging motor," said Michael Rossmann, the Hanley Distinguished Professor of Biological Sciences in Purdue's College of Science.
The article's lead author is Siyang Sun (pronounced See-Young Sun), a postdoctoral research associate working in Rossmann's lab. The researchers have proposed a mechanism for how the motor works.
Findings are detailed in a paper published Thursday (March 22) in the journal Molecular Cell. The paper was written by Sun; Kiran Kondabagil, a postdoctoral research associate at The Catholic University of America; Petra Gentz, a former visiting doctoral student at Purdue; Rossmann; and Venigalla B. Rao, a professor of biology at The Catholic University of America.
"The virus first assembles the protein shell of the head and then packages the DNA into this empty capsid," Rossmann said. "This process could be likened to building a house and then filling it with furniture."
The DNA is a complete record of a virus' properties, and the capsid protects this record from damage and ensures that the virus can reproduce by infecting a host organism.
"This research represents a significant step forward to understanding how DNA genetic material is packaged in viruses," Rao said.
Energy to run the packaging motor is produced when the ATPase enzyme breaks down a biological compound called adenosine triphosphate, or ATP, turning it into adenosine diphosphate, or ADP. Specifically, the enzyme breaks a bond between a chemical group called a phosphate and the ADP.
The researchers determined the structure of the particular ATPase used to break the high-energy bond that links the phosphate to ADP. This release of energy is used to run the molecular motor in the T4 virus.
The virus consists of a head and tail portion. The DNA-packaging motor is located in the same place where the tail eventually connects to the head. The motor falls off after the packaging step is completed, allowing the tail to attach to the capsid.
DNA is made of four different kinds of "nucleotides" identified by a specific "base." The bases are paired together to form the rungs of a ladderlike, double-stranded helical structure. Because there is a negative charge associated with each nucleotide, they repel each other when compressed together, creating a pressure inside the confining space of the capsid. A motor is needed to counteract this pressure, in effect pumping the DNA into the head.
The authors of the research paper have proposed a mechanism for how the motor works by comparing its structure to those of other, similar enzymes called helicases. The helicases are needed to separate double-stranded DNA into single strands during gene replication, a fundamental process of life required to pass on genetic information from one generation to another.
Helicases alternatively bind to and release their grip on DNA during replication, progressively moving along the helix to separate the strands in a motion similar to an inchworm's movement. The authors proposed that the motor uses a similar inchworm mechanism to package the DNA into the virus.
"While the helicases use the mechanism to unwind double-stranded DNA, this ATPase uses the mechanism to pump genetic material into the virus capsid," Sun said.
The researchers determined the enzyme structure using a technique called X-ray crystallography. This technique involves crystallizing a substance like the ATPase and then passing X-rays through the crystals, creating a "diffraction pattern" that can be interpreted with various computational procedures to produce an image.
Because herpes and other viruses contain similar DNA packaging motors, the findings could help scientists to design drugs that would interfere in the function of these motors and hence mitigate the result of some viral infections. The findings also could have other applications, such as development of tiny "nanomotors" in future machines, Rossmann said.
"But it's really premature to discuss possible practical applications of this knowledge because this work is really in the realm of basic research," Rossmann said. "When the Greeks observed the planets, they never thought such information would be helpful centuries later for designing spacecraft trajectories."
Written from a news release by Purdue University.