Some cells are meant to live, and some are meant to die. The linker cell of Caenorhabditis elegans, a tiny worm that is a favored model organism for biologists, is among those destined for termination. This cell helps determine the shape of the gonad in male worms--and then it dies, after two days, just as the worms are transitioning from larvae into adults. This programmed cell death is a normal part of the animal's development, yet the genetic and molecular mechanisms underpinning it have not been worked out.
Scientists in Rockefeller University's Laboratory of Developmental Genetics, headed by Shai Shaham, had previously shown that the linker cell does not expire by apoptosis, a more commonly studied form of programmed cell death. "Everything about this death process is different from apoptosis," he says. "It looks different under the microscope, it requires different genes, and it has different kinetics."
Many ways for cells to die have been observed and described in the artificial milieu of a tissue culture dish, but not in a living organism. Now, the Shaham lab has been able to study the molecular mechanism that causes linker cell death in worms. Their findings, reported in eLife, suggest that the linker cell's newly discovered dying process resembles that which leads to the loss of neurons, or neuronal parts, in people with some neurodegenerative disorders.
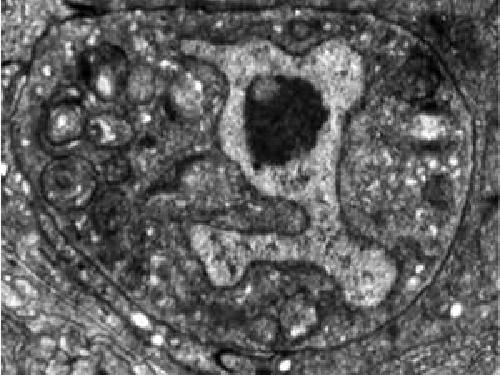 This dying worm linker cell, captured by electron microscopy, has features in common with neurons that expire prematurely due to neurodegenerative disease. Credit: The Laboratory of Developmental Genetics at The Rockefeller University/eLife
This dying worm linker cell, captured by electron microscopy, has features in common with neurons that expire prematurely due to neurodegenerative disease. Credit: The Laboratory of Developmental Genetics at The Rockefeller University/eLife
A new role for an old protein
To figure out the molecular processes that cause linker cell death, Shaham's team introduced mutations at random in worms and then searched for animals in which the linker cell survives for longer than normal. They identified a number of mutations that prolong the survival of linker cells, including one that affects the function of HSF-1, a protein known to shield cells from physiological stresses like heat.
"It was a big surprise that HSF-1, which typically plays a protective role in the cell, was found to be such a key regulator of this cell death," notes Shaham. His lab found that the protein performs two separate tasks in the cell that are independent from one another. So much so that when worms with a normal, functional HSF-1 were raised at high temperatures, their linker cells survived for longer than they normally do--presumably because the protein was kept busy protecting the cells from the heat, and hence failed to promote linker cell death.
HSF-1 kills the linker cell by activating specific components of a protein destruction machinery apparatus in the cell, called the ubiquitin proteasome system. Mutations in components of this machinery have been shown previously to influence the degradation of neuron extensions in Drosophila and mice, suggesting that the new worm pathway may be used broadly.
Programmed cell death in other systems
Apoptosis, one form of programmed cell suicide, is well described--scientists know which molecules induce it, which molecules suppress it, and the processes that take place in the cell as it occurs. However, blocking apoptosis in mice appears to have little effect on overall mouse development. "This is a surprising observation, given how prevalent cell death is during growth," Shaham notes. "It suggests that other means of killing cells likely exist that we know little about."
Non-apoptotic cell death is also seen in some disease states. In the current study, the researchers found that the process in which linker cells are culled during a worm's development resembles the way brain neurons die during normal development in mice, and in people with Huntington's disease and other neurodegenerative disorders. It is also reminiscent of the neuronal cell death seen when nerve cells get severed, as they do during spinal injuries.
Based on their recent findings in worms, Shaham and his coworkers hope to find out whether the human counterparts of the proteins promoting linker cell death in worms might be involved in neurodegeneration. If this turns out to be the case, these proteins might serve as targets for future drugs to slow the progression of Huntington's disease, or to help people regain mobility after a spinal injury.
"For example, if we stress the nerve cells while they are dying, so that the HSF-1 protein is forced to go into protective mode rather than cell killing mode, perhaps we can slow their death," speculates Shaham.
source: Rockefeller University