"Not only that, but the structure we observed matches the known structure of lysozyme and shows no significant sign of radiation damage, despite the fact that the pulses completely destroy the sample. This is the first high-resolution demonstration of the 'diffraction-before-destruction' technique on biological samples, where we're able to measure a sample before the powerful pulses of the LCLS damage it," he added.
The team chose lysozyme as the first sample for their research because it is easy to crystallize and has been extensively studied. Their work not only determined lysozyme's structure at such high resolution that it showed individual amino acids, but also proved the ability to use extremely small crystals for a range of applications. Boutet says the team has also studied more complex proteins and systems that they are analyzing now.
Ultimately, scientists using LCLS are driving toward an atomic- and molecular-scale understanding of complex biological systems -- such as the membrane proteins that are critical in cell functions and the mechanisms that power photosynthesis -- which could lead to discoveries in a range of sciences, from pharmaceutical breakthroughs to new sources of alternative energy.
The experiment was the first study performed on the new Coherent X-ray Imaging (CXI) instrument, a "molecular camera" designed, built and commissioned by SLAC and now available to the scientific community. Also key to the study was a novel custom-made detector, the Cornell-SLAC Pixel Array Detector (CSPAD), developed in collaboration between Cornell University and SLAC for use at the CXI instrument.
"This important demonstration shows that the technique works, and it paves the way for a lot of exciting experiments to come," says Boutet.

The ultimate goal of the CXI instrument is to perform imaging of single biological molecules as depicted in a simplistic manner in this movie. The single LCLS pulses travel to the CXI location in the Far Experimental Hall, where a simplified CXI instrument is shown, with a sample injector delivering single biomolecules to the LCLS beam. As we zoom in, we can see the molecules being hit by the LCLS beam, which destroys them, but not before the scattered X-rays are on their way to the detector, allowing a diffraction pattern from an undamaged molecule to be recorded. The individual diffraction patterns are recorded and stacked into the computer memory. Each image, coming from a different random orientation is then classified into bins corresponding to the orientation of the molecule. The different classes of orientations are averaged together and then the full 3D pattern is assembled. Once the 3D pattern is known, phase retrieval techniques allow the 3D atomic structure of the biomolecules to be deduced from the diffracted intensities alone.
(Photo Credit: SLAC National Accelerator Laboratory)
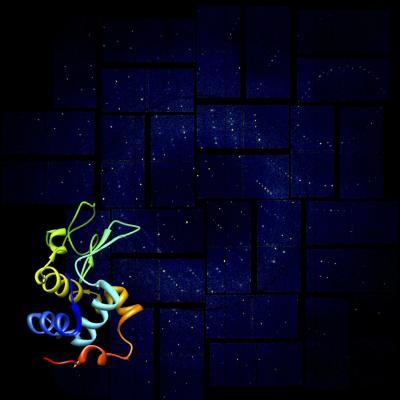
This rendering shows a lysozyme structural model against its X-ray diffraction pattern from SLAC National Accelerator Laboratorys Linac Coherent Light Source (LCLS), a powerful X-ray laser facility. Researchers have achieved high-resolution images of these simple biomolecules using advanced crystallography at LCLS. This successful demonstration paves the way for studies of more complex biological structures.
(Photo Credit: Anton Barty/DESY)