An international group of scientists working at the Department of Energy's (DOE) SLAC National Accelerator Laboratory has mapped a weak spot in the parasite that causes African sleeping sickness, pinpointing a promising new target for treating a disease that kills tens of thousands of people each year.
The results, reported Nov. 29 in Science Express, are already being enlisted in the effort to combat the disease, which is transmitted by tsetse flies infected with the single-celled parasite. The study also marks a milestone in using X-ray lasers, such as SLAC's Linac Coherent Light Source (LCLS), to determine the structures of biological molecules that are important for human health.
"This is the first new biological structure solved with a free-electron laser," said Henry Chapman of the Center for Free-Electron Laser Science in Hamburg, Germany, one of the leaders of the research team.
Lars Redecke, another team leader, said, "This is really a landmark in structural biology, and a significant step toward developing a new drug." Redecke is a structural biologist at the Joint Laboratory for Structural Biology of Infection and Inflammation of Hamburg and Lübeck universities in Germany.
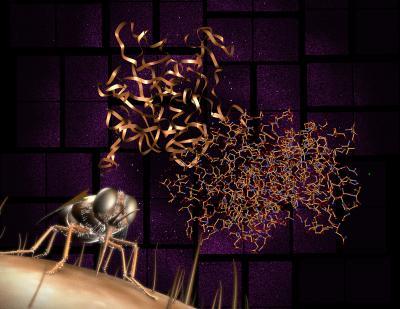
Researchers have revealed the detailed structure of an important protein involved in the transmission of African sleeping sickness. To do so, scientists used the LCLS X-ray laser and created diffraction patterns (shown in background) that were then reconstructed into the molecular structure (shown at center). The research is an important step toward developing a new drug to target the disease, which is carried by tsetse flies and is responsible for tens of thousands of deaths each year.
(Photo Credit: Greg Stewart / SLAC National Accelerator Laboratory)
About 60 million people across Africa are at risk for contracting African sleeping sickness, which kills an estimated 30,000 people each year. Existing drug treatments can be painful and cause serious side effects, and the parasites are becoming increasingly drug-resistant.
One of the parasite's main weapons is an enzyme that breaks down the proteins of its victims. Scientists hope to stop the disease by mimicking a natural "inhibitor" that keeps the enzyme in check until the parasite invades its victim's bloodstream. But the parasite's enzyme is so similar to one in humans that blocking it could also harm the patient, and researchers needed more detailed information about its structure to design a drug that attacks only the parasite.
Two recent developments allowed them to overcome this problem: a new way to grow crystals of the enzyme for analysis and the ability to analyze those tiny crystals with the LCLS X-ray laser, whose pulses are so intense and fast that they capture structural information before a sample is damaged.
The researchers grew the crystals inside live insect cells, freezing the enzyme in its natural inhibited state. Then they streamed the crystals into the laser's path, producing patterns in a detector that were used to reconstruct the enzyme and its inhibitor in 3-D and at nearly atomic scale.
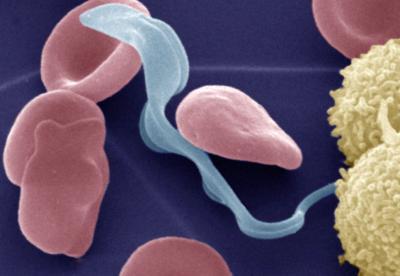
Colored electron micrograph of the bloodstream form of the Trypanosoma brucei parasite (light blue) that causes African trypanosomiasis (also called sleeping sickness) in humans in the presence of erythrocytes (red) and lymphocytes (yellow). After infection of the mammalian host by bite of the tsetse fly, the parasite lives within the bloodstream, before it invades the central nervous system and the brain to cause fatal effects.
(Photo Credit: Michael Duszenko / University of Tübingen)
"In my opinion, we provided the most complete blueprint available so far for the development of a synthetic inhibitor to block this enzyme," Redecke said. "Whether it will be successful is hard to say, but the odds are significantly increased by this structural data."
Redecke said his research team is working to crystallize proteins relevant to other parasites and viruses, including strains of hepatitis and flu, that could also be studied at LCLS, and he expects this field of research to grow.
"Our study will encourage others to use free-electron lasers to obtain new structural information of biologically relevant molecules," Redecke said.
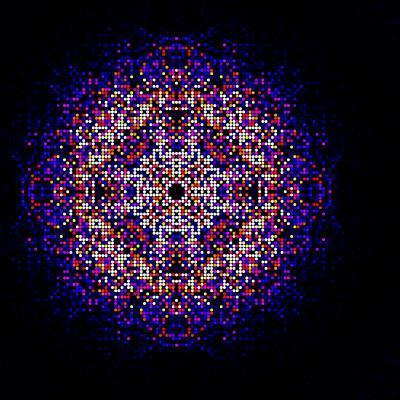
A map of intensities merged using the CrystFEL software suite from almost two hundred thousand diffraction patterns obtained from in vivo grown crystals of Trypanosoma brucei cathepsin B. This map is used to synthesize the three-dimensional molecular structure of the enzyme.
(Photo Credit: Karol Nass / CFEL)