Behind all motor, sensory and memory functions, calcium ions are in the brain, making those functions possible. Yet neuroscientists do not entirely understand how fast calcium ions reach their targets inside neurons, and how that timing changes neural signaling. Researchers at the Okinawa Institute of Science and Technology Graduate University have determined how the distance from calcium channels to calcium sensors on vesicles affects a neuron's signaling precision and efficacy. In international collaboration with research institutes such as the Pasteur Institute and the Institute of Science and Technology Austria, Professor Tomoyuki Takahashi and the Cellular and Molecular Synaptic Function Unit described the locations of voltage-gated calcium channels, which allow calcium ions to enter into the neuron, triggering vesicles to release neurotransmitters, signaling to the next neuron. This research, to be published the January 7, 2015 issue of Neuron, illuminates decades of mystery behind the precision and efficacy of neurotransmitter release, suggesting how signaling changes as an animal matures.
After an electrical spike, or an instantaneous change in voltage, travels through the neuron, it reaches the presynaptic terminal. The presynaptic terminal is an area facing the synaptic cleft, or the gap between one neuron and the next. The electrical spike triggers voltage-gated calcium channels to open, allowing calcium ions to enter the presynaptic terminal. The calcium ions then diffuse locally around the channels and encounter synaptic vesicles, small packages of neurotransmitters, which are signaling molecules. The calcium ions interact with sensor proteins on the vesicle, triggering the vesicles to fuse with the presynaptic terminal membrane, and releasing neurotransmitters into the synaptic cleft toward the next neuron.

Professor Tomoyuki Takahashi leads the Cellular and Molecular Synaptic Function Unit at OIST.
(Photo Credit: OIST)
Yet researchers have never fully grasped how calcium travels from gated channel to vesicle. Some researchers argued that the channels were spread across the active zone of the presynaptic terminal, while others argued that a ring of gated channels surrounded each vesicle. Therefore, Takahashi's project began with an electron microscope technique, where the researchers froze the presynaptic membrane and broke it open to expose the calcium channels. They found that the channels existed in clusters, with a variable number of channels in each cluster.
Next, the researchers ran various tests and simulations to determine how the channel clusters impact signaling. They found that clusters with more calcium channels more effectively trigger a nearby vesicle to release neurotransmitters. Importantly, channel clusters closer to vesicles trigger neurotransmitter release more quickly and more efficiently than clusters located farther from vesicles, increasing signal precision. "The calcium sensor on vesicles need a high concentration of calcium to trigger vesicle release," Takahashi said. "If the calcium entered from farther away, then it would diffuse into a lower concentration or bind to other proteins before reaching the calcium sensor on the vesicle."
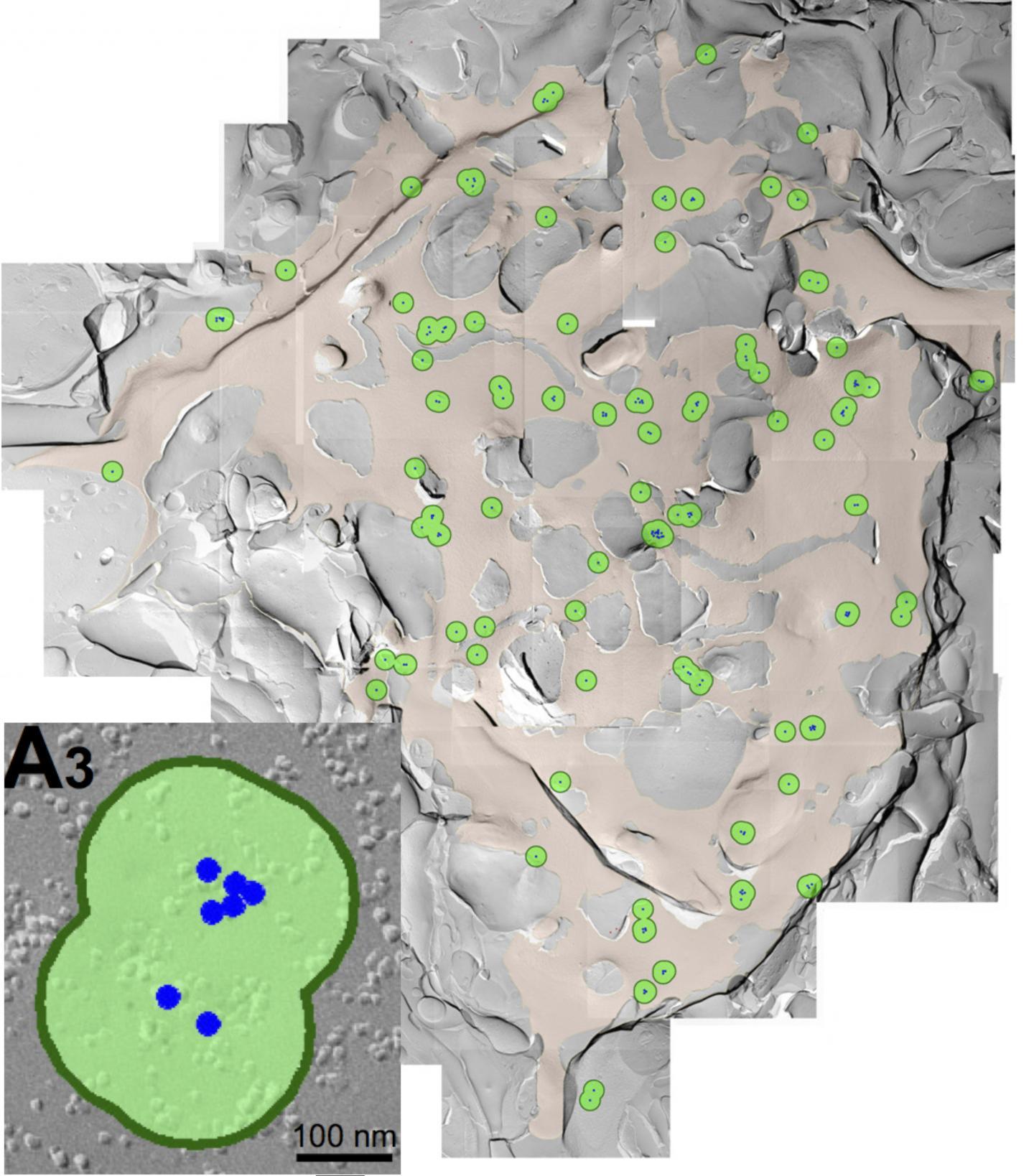
This is a freeze fracture replica image showing the voltage-gated calcium channels clusters on presynaptic membrane in rats. The green circles represent channel clusters, and inside each green circle are small black dots, which are the individual channels. This is easier to see in the inset, labeled A3, where the channels are blue dots.
(Photo Credit: OIST Professor Tomoyuki Takahashi)
Takahashi and his collaborators also studied how the distance changes as their rat subjects developed, and how the distance changes affect neural signaling. As the rat aged from seven days to fourteen days, the distance between the gated channels and the vesicle shrank from 30 nanometers to 20 nanometers. "This maturation is fairly significant," Takahashi said, explaining that the vesicles release much more quickly after calcium enters the synapse. "The signal becomes 30% faster," he said.
Moving forward, Takahashi and his collaborators propose the perimeter release model for use in neuroscience research. This model establishes that calcium channels exist in clusters and that the distance from these clusters to a vesicle is significant. "If you measure the distance from the center of the cluster, then this distance depends on the size of the cluster," Takahashi said. Therefore, the researchers propose the distance from vesicle to gated channel clusters be measured from the perimeter of the cluster, rather than the center. Distances calculated using this new model can explain how signaling precision increases during development.
"If there is anything which widens this distance," Takahashi said, "it actually interferes with neural precision and it can interfere with memory formation."
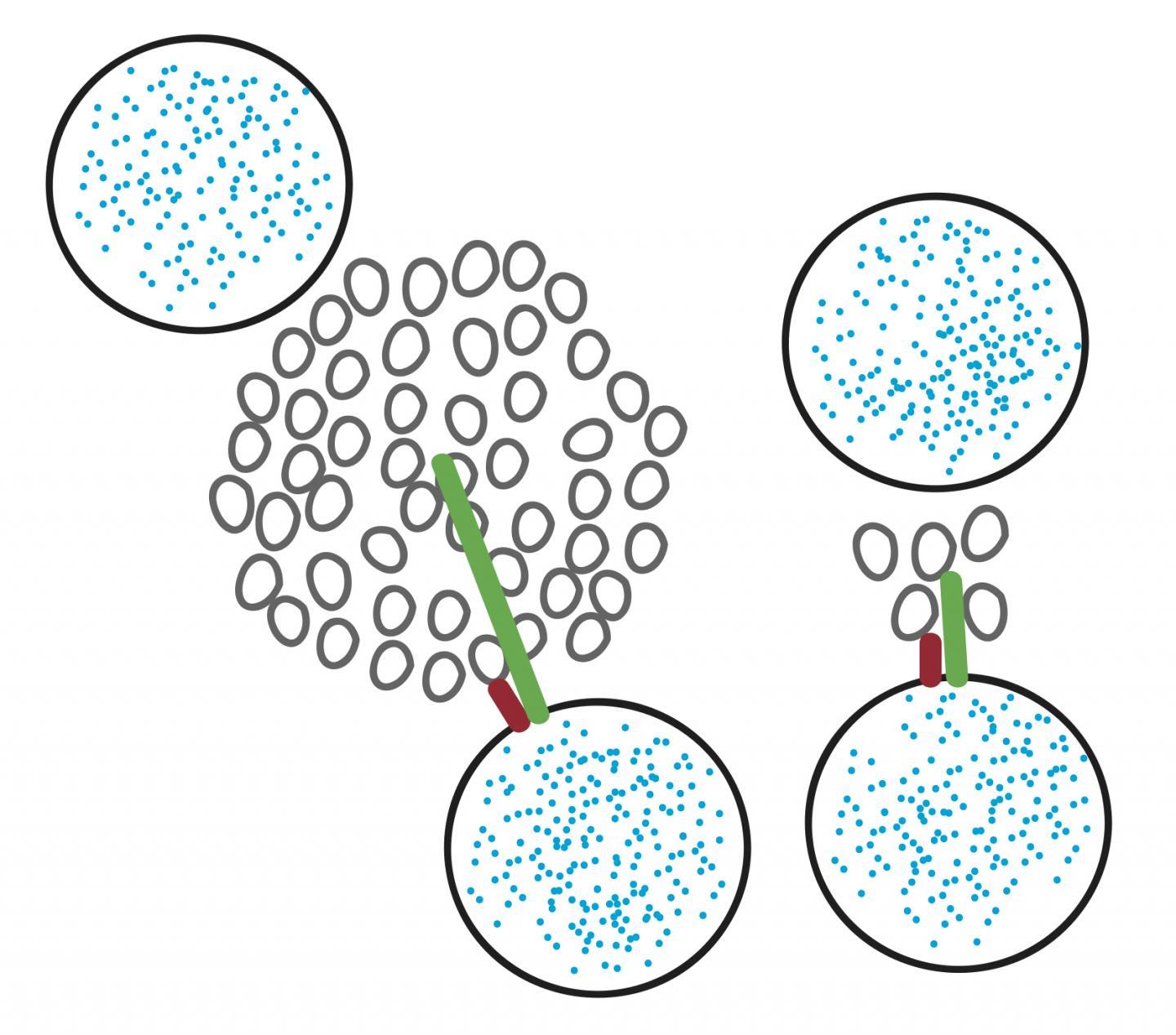
This diagram shows the new model that Professor Takahashi and his collaborators have proposed. Circles are vesicles filled with blue neurotransmitters, and the smaller grey circles are voltage-gated channels. Instead of measuring from the vesicle to the center of the channel cluster (green line), Takahashi suggests measuring to the perimeter of the channel cluster (red line). The difference is that measuring to the center varies with the size of the cluster, whereas measuring to the perimeter will always describe the closest channel to the vesicle
(Photo Credit: OIST)
Source: Okinawa Institute of Science and Technology (OIST) Graduate University