This discovery has a potential application in the treatment of certain blood disorders for which there is currently no cure. The study was led by Dr. Simón Méndez-Ferrer of the CNIC, working in partnership with the laboratories of Doctors Jürg Schwaller and Radek Skoda of the University Hospital in Basel (Switzerland). The study's authors have demonstrated in mice that tamoxifen, a drug already approved and widely used for the treatment of breast cancer, blocks the symptoms and the progression of a specific group of blood disorders known as myeloproliferative neoplasms.
Dr. Méndez-Ferrer explains that scientists have known for some time that men have a higher risk than women of developing leukemia: "We didn't know the causes off this different incidence of leukemia between men and women, but sex hormones like estrogen could at least partly explain these differences." Although estrogens were known to regulate some types of blood cells, very little was known about their influence on blood stem cells, including those that cause myeloproliferative neoplasms.
From this starting point, the researchers discovered an important practical application. "In this study we demonstrate that tamoxifen has specific effects on certain cells in the bone marrow, the hematopoietic stem cells and their immediate descendants, known as multipotent progenitors," explains study author Abel Sánchez-Aguilera.
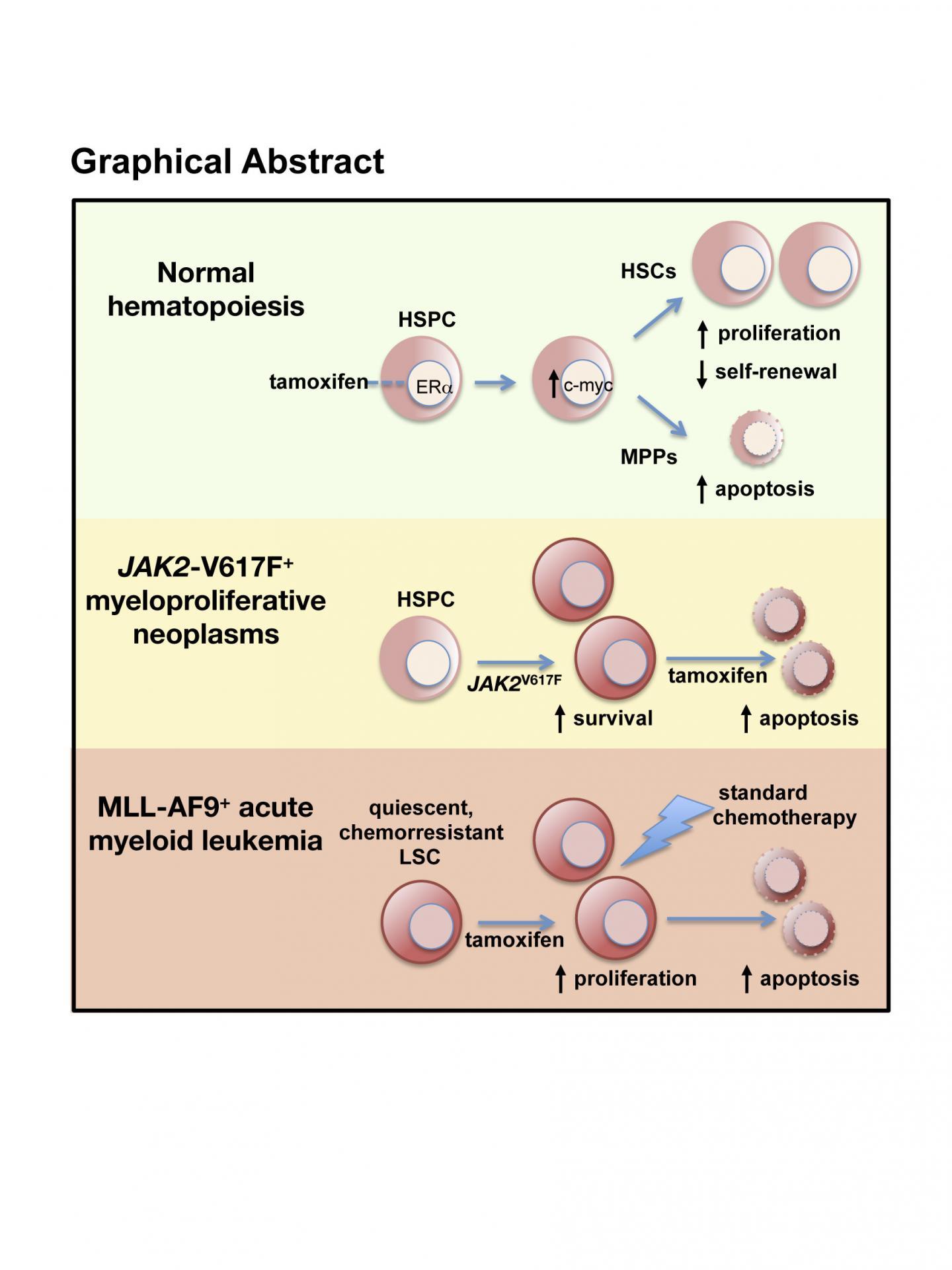
This is a graphic of the study. This discovery has a potential application in the treatment of certain blood disorders for which there is currently no cure.
(Photo Credit: CNIC)
The researchers found that tamoxifen had very distinct effects depending on whether the mice were healthy or sick. When administered to healthy animals, tamoxifen induced cell death of the multipotent progenitors, whereas the stem cells accelerated their division and partially lost their functionality. But when tamoxifen was administered to sick animals, symptoms disappeared and disease progression was blocked. In short, an effective therapy.
And there's another advantage. Surprisingly, these effects cause hardly any discernable alteration in the rest of the blood cells, which are maintained at normal levels even after prolonged treatment with the drug, showing no appreciable toxicity. Unlike the situation in breast cancer, where tamoxifen blocks the action of estrogens, in blood cells the team found that the drug acts by imitating the function of the hormone.
Myeloproliferative neoplasms, like polycythemia vera, are frequent tumors caused by a mutation in the gene that produces the protein JAK2 in hematopoietic stem cells. These diseases currently have no effective cure apart from bone marrow transplant, which is only possible for a small fraction of patients. The disease causes an accumulation of abnormal blood cells and the degeneration of the bone marrow, and in mice both these processes are blocked by treatment with tamoxifen. The treatment is able to eliminate the abnormal stem cells, the root cause of the disease, something that current therapies, including JAK2 inhibitors, don't manage.
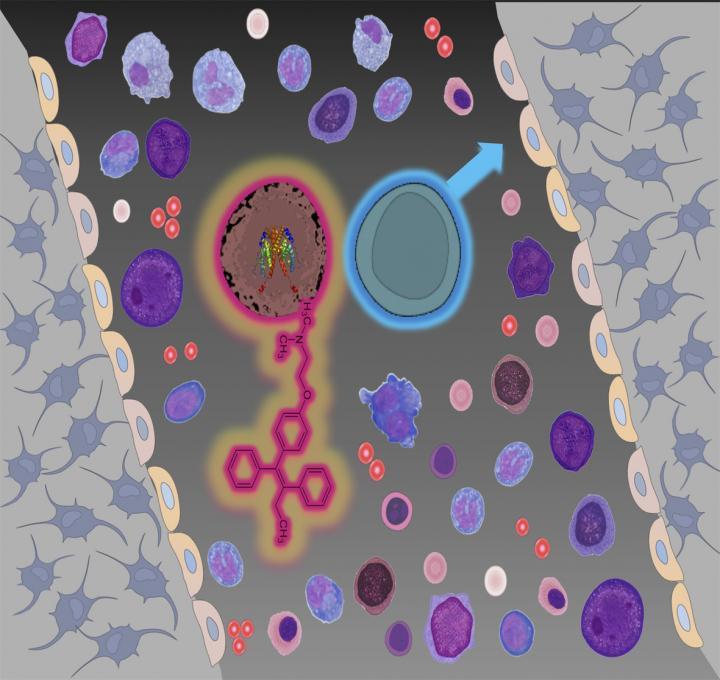
This study suggests that the blood stem cells (pink for female, blue for male) that, when altered, produce excessive blood cells (diseases called neoplasms) can be killed by female sex hormones. This finding potentially explains why these diseases and related cancers are more common in men than in women. The approved drug tamoxifen, whose chemical structure resembles female sex hormones and has been used in the picture to depict the feminine symbol (pink), can be used to block the development of blood neoplasms in male and female mice.
(Photo Credit: Andrés García-García)
Dr. Simón Méndez-Ferrer points out that "although we don't know yet exactly why, tamoxifen appears to have a stronger effect on leukemic cells than on the healthy ones, which allows the disease to be blocked without producing major secondary effects on normal blood cells."
One of the most remarkable features of this study is its potential for translation to clinical practice in a relatively short time. The study's lead author concludes, "The fact that tamoxifen is already approved for clinical use and has an appropriate safety profile enormously increases the chances of these results leading to a clinical trial to test this potential therapy in patients with myeloproliferative neoplasms."

This image shows Simon Mendez - Ferrer's team.
(Photo Credit: CNIC)