A microchip-based device developed by Massachusetts General Hospital (MGH) investigators may greatly simplify the monitoring of patients' response to treatment for ovarian cancer – the most lethal form of gynecologic cancer – and certain other malignancies. The team from the MGH Cancer Center and the Center for Systems Biology reports using their device to isolate and identify tumor cells from ascites, an accumulation of fluid in the abdomen that often occurs in abdominal cancers. The PNAS Early Edition paper also describes development of a panel of four protein markers to accurately identify ovarian cancer cells in ascites.
"We were able to demonstrate that simply squirting small amounts of otherwise discarded ascites fluid into our device allowed us to quantify tumor cells and explore mechanistic markers of tumor progression without the need to process liters of ascites with advanced instrumentation not readily available in many community hospitals," says Cesar Castro, MD, MMSc, MGH Cancer Center and Center for Systems Biology, co-lead author of the PNAS paper. "Moreover, achieving point-of-care readouts of tumor cell markers from repeatedly collected ascites at different time points, could allow for frequent monitoring of treatment response without having to wait for the next imaging scan."
The ability to reliably track treatment response essentially lets caregivers know whether a particular anticancer drug should be continued or if another option should be tried. Tumor recurrence begins before metastases become visible on imaging studies, so several options for non-invasive "liquid biopsies" are being investigated, including analysis of circulating tumor cells and other factors found in the blood. Since ovarian cancer metastases are usually confined to the abdominal cavity and ascites commonly form in advanced disease, the research team theorized that ascites fluid could be an alternative, if not better, option than blood for treatment monitoring.
Isolation of ascites tumor cells (ATCs) has been challenging, since they constitute less than 1 percent of the cells in ascites fluid. ATCs themselves vary greatly in size, and other fluid contents – inflammatory and blood cells, cells from the abdominal lining and additional debris – often form large clumps that would clog typical microfluidic devices. Along with removing the non-tumor-cell components of ascites fluid, the team also needed a way to accurately identify ovarian cancer cells and analyze their molecular properties.
Through a lengthy process that involved laboratory work comparing ovarian cancer cells with benign cells and ascites samples from ovarian cancer patients with those from individuals with noncancerous conditions like cirrhosis, the investigators uncovered a panel of four protein markers that specifically identified ATCs from ovarian cancer patients. They confirmed the accuracy of the panel, called ATCDX, in two separate sample sets, comparing ascites fluid from ovarian cancer patients with either noncancerous fluid or with ascites from patients with other types of cancer.
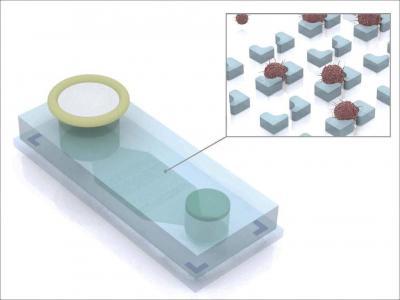
Ascites fluid from ovarian cancer patients is introduced through a filter into an inlet (left) where magnetically labeled benign cells are trapped. The remaining fluid passes through a microchip containing microwells where antibody-labeled tumor cells are captured (inset) for collection, imaging, and additional analyses.
(Photo Credit: Hakho Lee, PhD, and Jaehoon Chung, PhD, MGH Center for Systems Biology)
Prior to the passage of ascites fluid through the MGH team's device – called the ATC chip – the sample is first labeled with magnetic nanoparticles that bind to noncancerous inflammatory cells. The sample is introduced into the three-inch-long ATC chip through a filter that screens out clumps of debris and then passes by a magnet that traps the magnetically labeled benign cells. Also added to the device is a mixture of antibodies to the ATCDX proteins, which label the markers for imaging detection. After the magnetic sorting, the sample passes over a series of microwells of successively smaller size, which collect ATCs while even smaller leukocytes pass through the device. The concentration of ATCs captured on the chip is 1,000 times greater than it was in the original fluid sample.
The investigators tested the device initially by analyzing ascites samples repeatedly collected from a single ovarian cancer patient over a 14-week course of treatment, first with standard chemotherapy and then with antiangiogenic therapy when disease progression resumed. The ATC chip revealed that the number of ATCs dropped during initial treatment response, rose with progression and fell again as antiangiogenesis relieved the patient's symptoms. They also found that analysis of the molecular properties of ATCs from 46 ovarian cancer patients could distinguish those whose tumors responded to treatment from nonresponders.
"This device far exceeded our expectations," says Ralph Weissleder, MD, PhD, director of the Center for Systems Biology and senior author of the PNAS report. "Coupled with our diagnostic panel, we were able to clearly distinguish between tumor cells and the extensive cellular debris commonly found in ascites. The ATC chip and the set of protein markers we uncovered, which reliably identified ovarian cancer cells floating in ascites, provide a novel platform for extending ATC analysis to settings where the expensive equipment and labor-intensive techniques that ATC isolation previously required would not be feasible."
The research team notes that large-scale production of the ATC chip is already being planned, and if future studies confirm their results, the device's low cost – estimated at less than $1 each – and ease of use would make ATC analysis a practical and valuable tool for both treatment of and research into ovarian cancer and possibly for other tumors that induce the formation of ascites, including pancreatic cancer.
Source: Massachusetts General Hospital