Researchers from North Carolina State University have created a range of soft, elastic gels that change color when exposed to ultraviolet (UV) light – and change back when the UV light is removed or the material is heated up.
The gels are impregnated with a type of photochromic compound called spiropyran. Spiropyrans change color when exposed to UV light, and the color they change into depends on the chemical environment surrounding the material.
The researchers made the gels out of an elastic silicone substance, which can be chemically modified to contain various other chemical compounds – changing the chemical environment inside the material. Changing this interior chemistry allows researchers to fine-tune how the color of the material changes when exposed to UV light.
"For example, if you want the material to turn yellow when exposed to UV light, you would attach carboxylic acid," explains Dr. Jan Genzer, Celanese Professor of Chemical and Biomolecular Engineering at NC State and co-author of a paper describing the research. "If you want magenta, you'd attach hydroxyl. Mix them together, and you get a shade of orange."
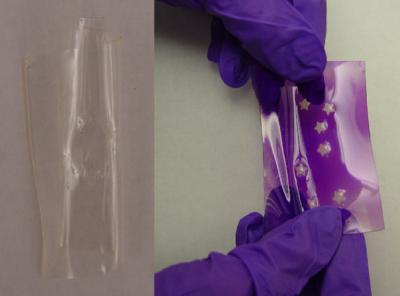
The image to the left shows one of the photochromic gels before exposure to UV light. The image on the right shows the elastic material after exposure to UV light.
(Photo Credit: Jan Genzer, North Carolina State University)
Photochromic compounds are not new, but this is the first time they've been incorporated into an elastic material, without impairing the material's elasticity.
The researchers were also able to create patterns by using a shaped mold to change the chemical make-up of specific regions in the material. For example, applying hydroxyl around a star-shaped mold (like a tiny cookie cutter) on the material would result in a yellow star-shaped pattern appearing on a dark magenta elastic when it is exposed to UV light.
"There are surely applications for this material – it's flexible, changes color in UV light, reverts to its original color in visible light, and can be patterned," Genzer says. "At this stage we have not identified the best application yet."
Source: North Carolina State University